MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Structure solution of a new ordered mixed imide-amide compound for hydrogen storage
E. Napolitano1, F. Dolci1, R. Campesi1, C. Pistidda2, M. Hoelzel3, P. Moretto1, and S. Enzo4
1Institute for Energy and Transport, European Commission – DG Joint Research Centre, Petten, the Netherlands
2Institute of Materials Research, Helmholtz-Zentrum Geesthacht, Geesthacht, Germany
3Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
4Dipartimento di Chimica e Farmacia, Università degli Studi di Sassari and INSTM, Sassari, Italy
In order to elucidate the reaction pathways in complex hydrogen storage materials, the compound KMg(NH)(NH2) was synthesized from the reversible dehydrogenation process of a Mg(NH2)2/KH mixture. Preliminary powder X-ray diffraction patterns on specimens without any deuteration were supplemented with neutron powder diffraction studies on the reaction products from deuterated precursors with the intent of solving the crystal structure. The compound presents the orthorhombic space group Pnma (62) with lattice parameters: a = 9.3497(3)Å; b = 3,6631(1)Å; c = 9.8901(3)Å, respectively. The coexistence of imide/amide groups in the same compound allows us to notice for the first time a heptahedral geometry arrangement around potassium atoms by imide and amide units.
In the field of solid-state hydrogen storage, the alkali amides and alkaline-earth analogues show remarkable reversibility in terms of hydrogen release and up-take processes [1]. Unfortunately, the formation of new unknown phases during intermediate steps of the absorption/desorption reaction pathways may constitute a severe limitation to understanding the basic mechanisms of the reaction kinetics. Recently, the formation of a new unknown KMg(NH)(NH2)compound was reported by Wang et al. [2], unfortunately without its crystal structure being established.
In such cases, where compounds with hydrogen atoms are concerned, deuteration and neutron powder diffraction patterns combined with the so-called ab-initio [3] approach appear well suited to shed light on the hydrogen atom interactions and to solve the complete structure of an unclassified new compound.
Experimental methods and numerical data analysis
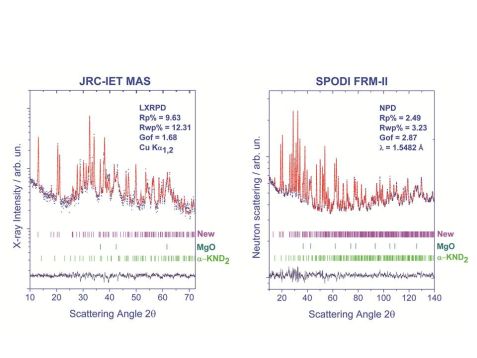
The KMg(ND)(ND2) compound was prepared applying the synthetic procedure reported by Wang et al.2. The deuterated reagents were produced as described by Napolitano et al. [4]. Two experimental diffractograms were collected in order to solve the crystal structure of the unknown phase: a laboratory X-Ray powder diffraction pattern (JRC-IET MAS, Cu Kα, RT), collected in a Bragg-Brentano setting, and a Neutron powder diffraction pattern carried out in transmission geometry at the SPODI instrument (MLZ, λ = 1.5481 Å, RT) Fig.1. Preliminary phase identification analysis was conducted using X’Pert Highscore [5] software and the indexing step using the McMaille program [6]. The crystal structure model was carried out using the ab-initio methods applying the Direct-Space approaches [7] using Endeavour [8] software and the Maud [9] program for the Rietveld refinement.
The X-Ray and neutron diffraction patterns were subjected to parallel data treatment. The phase identification analysis revealed the presence of minor polycrystalline compounds: α-KNH2 and MgO. The remaining unassigned peaks were indexed in the orthorhombic crystal system with space group Pnma and lattice parameters: a = 9.3497(3) Å; b = 3,6631(1) Å; c = 9.8901(3) Å [10]. Starting from the assumption that the chemical composition of the unclassified phase was KMg(ND)(ND2), based on previous reported infrared results [2], the Direct-Space Method was applied in order to the unknown crystal structure. A total of 28 atoms were placed inside the unit cell, consisting of 4 asymmetric units and a calculated density of 1.91 g/cm3. During the final Rietveld refinement, microstructure and structure parameters were optimized, yielding the best fit with satisfactory residual values (Fig. 1- Tab. 1).
Crystal structure description
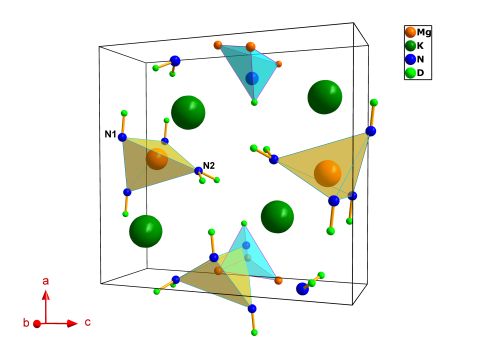
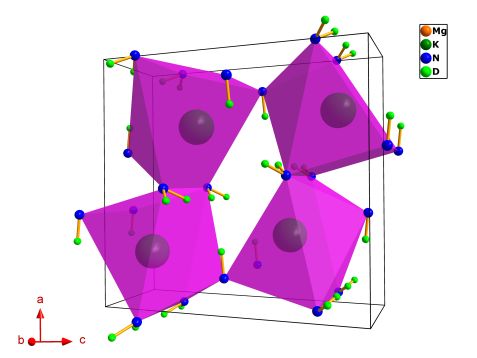
The crystal structure of KMg(ND)(ND2) composite contains features of both alkali and alkali metal earth imides and amide compounds. Each Mg2+ cation is tetrahedrally coordinated by three ND2- imide units (N1 atoms) with an Mg-N1 average distance of ca. 2.1 Å and one amide ND2- group (N2 atom) with Mg-N2 distance ca. 2.05 Å. Every N1 atom adopts a distorted tetrahedral coordination sphere by three Mg cations and one D1 atom (Fig. 2). The potassium cations experience a heptahedral coordination environment creating a trigonal prismatic mono-capped polyhedron with K-N distances ranging from 2.94 Å to 3.1 Å. Also, five of the eleven deuterium atoms present a distance K-D < 3 Å, showing the key role of potassium cations in sustaining the activation of N-D bonds (Fig. 3).
In this work, we report the crystal structure solution of the mixed imide-amide potassium-magnesium composite using X-Ray and Neutron powder diffraction data.
The KMg(ND)(ND2) compound presents imide and amide groups coexisting and interacting interacting with magnesium atoms in tetrahedral coordination, and shows for the first time a imide/amide heptahedral coordination arrangement around the potassium atoms.
References:
[1] P. Chen et al., Nature 420, 302 (2002).
[2] J. Wang et al., ChemSusChem 4,1622 (2011).
[3] K. D. Harris et al., Angew. Chem. Int. Ed. 40, 1626 (2001).
[4] E. Napolitano et al., Int. J. Hydrog. Energy 39, 868 (2014).
[5] PANalytical. X’pert Data Collector and X’pert Highscore. PANalytical BV, Almelo, The Netherlands (2003).
[6] A. Le Bail, Powder Diffr. 19, 249 (2004).
[7] R. Černý and V. Favre-Nicolin, Z. Kristallogr. 222, 105 (2007).
[8] Endeavour – Crystal Impact – www.crystalimpact.com/endeavour.
[9] L. Lutterotti The MAUD Program, www.ing.unitn.it/~maud/
[10] E. Napolitano et al., Int. J. Hydrog. Energy 39, 8181 (2014).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien: