MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
The effect of organic matter on mineral scaling of membranes for desalination of wastewater
V. Pipich1, Y. Dahdal2, H. Rapaport3, R. Kasher2, Y. Oren2, and D. Schwahn4
1Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
2Zuckerberg Institute for Water Research, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
4Heinz Maier-Leibnitz Zentrum (MLZ) and Physik-Department E13, Technische Universität München, Garching, Germany
A consequence of high demand for potable water in arid areas such as the Mediterranean is the increasing number of desalination plants using membrane-based reverse osmosis (RO) and nanofiltration (NF) technologies. A serious problem is membrane biofouling and scaling. Small-angle neutron scattering reveals the strong influence of organic molecules on mineralization. This leads to the conclusion that scaling could be minimized if organic molecules were not present [1].
Potable water in arid areas
In 2000, Israel initiated a “desalination master plan” with the aim of producing potable water at the rate of approximately 650 million m3 per annum from seawater by the year 2020 [2]. At present time, a decision was made by Israel, Jordan, and the Palestinian National Authority to divert 200 million m3 per annum of water from the Red Sea to the Dead Sea. A seawater desalination plant is planned near the Jordanian city of Aqaba to convert 40 % of this water to potable water to be shared between Jordan and the city of Eilat (Israel) [3]. Desalination plants are large factories, as demonstrated in figure 1 which shows part of the RO membrane units of the Ashkelon seawater desalination plant. In October 2013, the largest desalination plant in Sorek, south of Tel Aviv, became operational with a capacity of 624,000 m3 per day catering for about 10 % of the country’s drinking water consumption [2]. Despite this extensive activity, water supply in many countries, especially those that are far from the sea, is a serious problem. In these cases, recycling of impaired water such as municipal wastewater, upgraded to the level of unlimited application, is a reasonable solution. Another, no less important aspect of recycling is environmental protection. Membrane-based technologies are extensively used today in this respect.
Small-Angle Neutron Scattering as a tool for exploring biofouling and scaling
A serious problem of RO/NF desalination is biofouling and scaling of the membranes, which limits the efficiency of desalination and membrane lifetime. In particular, scaling by calcium phosphate is a severe problem as no reliable antiscalants (special agents for preventing scale formation) are currently known. Small-angle neutron scattering (SANS) may contribute valuable information to the mechanisms associated with these problems as neutrons distinguish between organic and inorganic phases and quantitatively analyze particles of size between nm and μm.
Protein induces mineralization
Mineralization has been explored in the presence of the two proteins bovin serum albumin (BSA) and lysozyme in a simulated secondary effluent (SSE). The SSE models the mineral fraction of an RO concentrate from the Shafdan Wastewater Treatment Plant (Israel) [4]. The two proteins are important examples of extracellular polymeric substances (EPS) forming biofilms and limiting the desalination efficiency of secondary wastewater effluents, particularly of RO membranes. Both proteins initiate a complex process of mineralization in SSE.
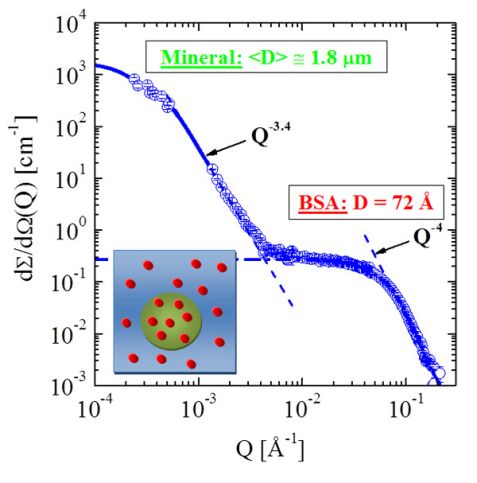
Scattering experiments of SSE-H2O and SSE-D2O solutions performed 4 days after preparation provided evidence of amorphous calcium phosphate (ACP) particles of 180 ± 16 Å diameter and of 8 · 10-6 volume fraction. Figure 2 shows a scattering pattern immediately after mixing BSA and SSE [1]. Strong scattering at small Q shows the formation of μm large particles of fractal structure with an exponent of (3.38 ± 0.06). This scattering is formed within a few minutes of mixing and remains stable for several hours. At large Q scattering from BSA monomers is visible, showing that only 20 % of the protein is involved in mineralization. Those experiments performed with varying content of SSE-H2O and SSE-D2O (thereby changing the scattering contrast) identified the μm large particles as protein-mineral-particles (PMP) of about 40 % organic volume fraction, which also explains the fractal exponent. A schematic representation of a PMP is shown in the inset of figure 2; protein-mineral composites (green circle) exist beside protein monomers (red dots). The volume fraction of the PMP’s is determined as 2 · 10-4 which is too large for calcium phosphate to be the only mineral composite. Investigating the solution product of possible mineral polymorphs in SSE, the formation of hydroxyapatite and of calcium carbonate PMP’s is suggested.
The SANS data clearly show that mineralization is minimized in the absence of organic molecules. Another strategy could be to enforce calcium phosphate and carbonate mineralization in the effluent by introducing organic molecules and filtering them out by subsequent UF/NF treatment, i.e. to eliminate phosphate and carbonate by coagulation.
This work was funded by the Ministry of Science, Culture and Sport (MOST) and the Bundesministerium für Bildung und Forschung (BMBF).
References:
[1] V. Pipich et al., Langmuir 29, 7607 (2013).
[2] https://www.water-technology.net/projects/sorek-desalination-plant/
[3] Frankfurter Allgemeine (FAZ): „Wasser für das Tote Meer“ vom 10.12. (2013).
[4] Z. Steiner et al., Environ. Sci. Technol. 44, 7937 (2010).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien: