MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Structure of metallo-supramolecular micellar gels
C. Mugemana1, A. Joset2, P. Guillet3, M.-S. Appavou4, N. De Souza5, C.-A. Fustin1, B. Leyh2, and J.-F. Gohy1
1Institute of Condensed Matter and Nanosciences (IMCN), Bio- and Soft Matter division (BSMA), Université catholique de Louvain, Louvain-la-Neuve, Belgium
2Department of Chemistry, Molecular Dynamics Laboratory, University of Liège, Sart-Tilman, Belgium
3Equipe Chimie Bioorganique et Systèmes Amphiphiles, Université d’Avignon et des Pays de Vaucluse, Avignon, France
4Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
5Bragg Institute, Australian Nuclear Science and Technology Organisation, Kirrawee, Australia
SANS analysis of metallo-supramolecular micellar gels from polystyrene-block-poly(tert-butylacrylate) PS-_b_-PtBA-diblock copolymers is presented. The solubilization of the PS-b-PtBA-tpy diblock copolymer in ethanol-d6 gives micelles formed by a polystyrene core and surrounded by PtBA coronal chains bearing terpyridine at the chain end. Adding metal ions to generate a micellar network gives rise to a micellar gel whenever the metal-ligand complexes are stable enough to strengthen the network. A significant neutron scattering signal is thus generated due to the contrast between the non deuterated copolymer micelles and deuterated ethanol.
Synthesis and self-assembly of PS-b-PtBA-terpy diblock copolymer
A PS70-b-PtBA180-tpy copolymer was synthesized by nitroxide-mediated radical polymerization. This copolymer was dissolved in deuterated ethanol, a selective solvent for the PtBA block, and a non-solvent of the PS block. The characteristic features of the resulting micellar solutions in the dilute regime were first investigated using dynamic light scattering at a concentration of 0.05 g L-1. DLS analysis showed well-defined objects with a hydrodynamic radius of 25.8 nm (PDI as determined by DLS: 0.06).
Neutron scattering intensities of micellar solutions loaded with metal ions
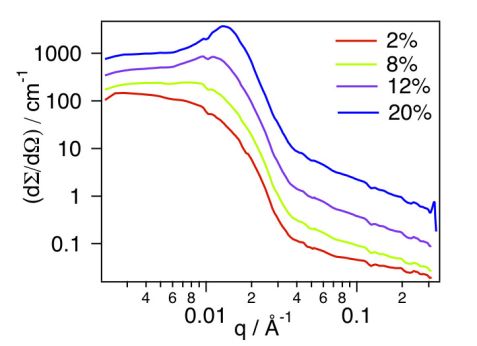
Figure 1 shows the scattered intensities obtained by SANS on micellar solutions in deuterated ethanol at 2, 8, 12 and 20% w/v concentrations. At the lowest concentration investigated (2% w/v), the macroscopic scattering cross section, dΣ/dΩ (q), decreases monotonically, suggesting that only isolated micelles are observed in this concentration regime, and thus giving access to the form factor of the micelles. In contrast, by increasing further the concentration to 8, 12 and 20% w/v, the scattering profiles revealed more and more clearly a structure factor peak arising from mutual interferences between the neutron waves scattered by different micelles.
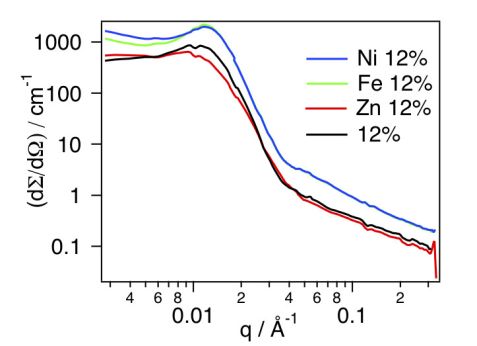
The impact of the addition of metal ions on the structure and spatial organization of the micelles was further investigated by adding of 0.5 eq. (with respect to the concentration of terpyridine ligands) of each metal ion, i.e. Fe(II), Ni(II) and Zn(II), to the micellar solutions. The resulting scattering cross sections are plotted in figure 2 for the micellar solutions at a concentration of 12% w/v. In the case of the micellar solutions dosed with Fe(II) and Ni(II) ions, an increase in the intensity of the structure factor peak in the low q regime was observed compared to the micellar solution with no metal ion. Such an increase is most likely to result from the formation of a network of micelles connected via metal-terpyridine complexes, since Ni(II) and Fe(II) ions preferentially form bis-terpyridine complexes. In the case of Zn(II), no structure factor peak was observed since Zn(II) forms, rather, mono-terpyridine complexes, which do not lead to a network of micelles, in agreement with previous results from rheology experiments [1].
Pedersen-Gerstenberg and Percus-Yevick models
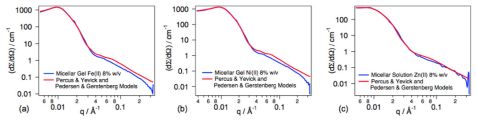
The internal structure of the micelles, from diluted to concentrated solutions, were studied using the Pedersen-Gerstenberg model [2]. The impact of the metal ions on the formation of a micellar network was further investigated. To this end, the micellar solutions were exposed to three metal ions, i.e. Ni(II), Fe(II) and Zn(II). These transition metals form complexes with terpyridine ligands exhibiting various binding strengths. The neutron scattering data of the micellar gels were analyzed using the models of Percus-Yevick for the structure factor and of Pedersen-Gerstenberg for the form factor. The experimental and the fitted neutron scattered intensities of the gels obtained upon addition of metal ions to the PS70-_b_-PtBA180-tpy micellar solutions are shown in figure 3.
The addition of 0.5 eq. of Ni(II) or Fe(II) to the 8% w/v micellar solution leads to the formation of a network since these ions form stable complexes with terpyridine ligands. An increase in the copolymer volume fraction is observed, corresponding to a contraction of the system which becomes a gel. Volume fractions similar to those obtained for the 12% w/v micellar solution without metal ion are reached. Interestingly, the hard sphere interaction distance, σ, is not affected, indicating that the network is consistent with micelles interconnected via metal-terpyridine complexes, without, however, interpenetration of the coronal chains that may lead to coronal chain entanglements.
Conclusion
SANS appears to be a powerful tool for investigating such complex systems based on supramolecular micellar gels by providing an insight into the inner structure and the spatial organization of the micelles.
References:
[1] P. Guillet et al., Soft Matter 5, 3409 (2009).
[2] C. Mugemana et al., Macromol. Chem. Phys. 214, 1699 (2013).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien: