MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Revision of the lithium intercalation into graphitic carbons
O. Dolotko1,2, A. Senyshyn1, M.J. Mühlbauer1,3, and H. Ehrenberg1,3,4
1Materials Science, Technische Universität Darmstadt, Darmstadt, Germany
2Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
3Institute for Applied Materials-Energy Storage Systems, Karlsruhe Institute of Technology, Eggenstein-Leopoldshafen, Germany
4Helmholtz-Institute Ulm for Electrochemical Energy Storage, Karlsruhe, Germany
Despite the common use of graphite as the anode in lithium-ion cells, the mechanism of lithium intercalation into graphite is still not completely understood. The evolution of the graphite structure in commercial Li ion cells has been studied in situ by using a combination of electrochemistry and high-resolution neutron powder diffraction. The phase diagram for lithiated carbons has been reconsidered and alternative ordering models for the Lix<0.5C6 have been proposed. Up to seven new phases of Lix<0.5C6 type have been detected during cell charge/discharge.
Graphite anode
Nowadays, graphite is the most commonly employed anode material in Li-ion batteries, its general intercalation feature being the formation of LixC6 compounds, called stages. Of the different stages, crystal structure models are known for LiC6 (stage I) and LiC12 (stage II) only. There have been long standing debates regarding the lattice metrics and atomic arrangement in LiC18 (IIL stage), while there have been no reports on higher-ordered lithium intercalated carbons such as stages III, IV, VIII, or I’. One reason for this lack of knowledge is the limited ability of X-ray to localize light elements for a precise structure determination. “In-operando” neutron scattering characterization of the entire cell during electrochemical cycling is a promising approach, providing unique information about processes occurring inside the cell, including localization of Li atoms. In situ investigation also eliminates any risks of material oxidation, electrolyte evaporation or cell charge changes.
Lithiation into graphite
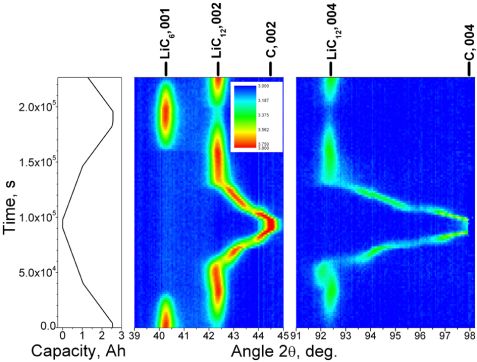
A cylindrical LiCoO2|C| based Li-ion cell of 18650-type was studied “in-operando” at the high-resolution neutron powder diffractometer SPODI (λ = 2.53633(1) Å). In contrast to the LiCoO2, which remains isostructural during the cell cycling [1, 2], a sequence of phase transformations is observed for the lithium intercalation into graphite. Stage I (LiC6) forms when the cell is charged above 1400 mAh, ∼0.5Qmax. This is the lithium intercalated carbon hosting the maximum amount of lithium (at ambient pressures). Its crystal structure resembles the structure of graphite consisting of hexagonal graphene sheets with A-A-stacking in c [3]. The structure of the stage II (LiC12) is very similar but only every second lithium layer is occupied, resulting in a doubling of the lattice parameter c and a slight reduction of the (002) interatomic spacing compared to the (001) of LiC6 due to A-A-B-B-A-A-… stacking.
The complicated non-continuous transformation of LiC12 into graphite consists of different stages and shows a clear asymmetry between discharge and charge (fig. 1). The structural similarity of the higher ordered stages makes their identification non-trivial. In order to reveal subtle changes, a single profile decomposition technique has been applied to the second order diffraction signals from (004) LiC12 to (004) C. Assuming the LiC6-to-LiC12
transformation occurring at xLi = 0.5, the lithium content in other phases can be quantified from the cell capacity.
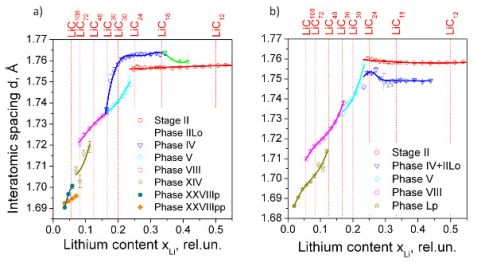
The analysis of interatomic spacings and intensities revealed the presence of lithiathed carbons with well-defined separation. This is shown in terms of a lithium-carbon phase diagram (fig. 2a and 2b). While the evolution of stages I and II indicates stability of their interatomic spacings with minor deviations, the higher-ordered lithiated carbons, termed as “phases”, display a Vegard behaviour instead of forming well-defined “stages”. Phases LiC6N, with integer N close to IV, V, VIII have clearly been observed, whereas no traces of phase III corresponding to LiC18 have been noticed. Instead of LiC18, a diffuse and broad signal has been observed as a tail on the LiC12 peak. It is more pronounced during discharge with a maximum at xLi = 0.379 and corresponds to a LiC16 composition (stage IIL). In total, two stages and four phases were identified during charging, while for the discharge two stages and seven phases were observed.
Lithium-graphite twisted bilayer behaviour
The observation of peaks having non-zero h and k indices revealed a formation of superstructure in a narrow capacity range. After checking different superspace index/modulation vector configurations, the best agreement was found to be with the modulation vector k = (α,α,0), on reducing the α upon lithium extraction. Such superstructural modulation may indicate that the mechanism of lithium intercalation into graphite is different from the commonly-accepted stage formation. An alternative structural model has been proposed where the observed modulations can be associated with a certain twist of graphene layers producing regions with A-A and A-B (B-A) type of graphene stacking, with the A-A arrangement of graphene layers being the obvious requirement for lithium intercalation [3].
References:
[1]A. Senyshyn et al., J. Power Sources 203, 126 (2012).
[2]O. Dolotko et al., J. Electrochem. Soc. 159, A2082 (2012).
[3]A. Senyshyn et al., J. Electrochem. Soc. 160, A3198 (2013).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien: