MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Low temperature oxygen diffusion in (Nd/Pr)2NiO4+δ explored using single crystal neutron diffraction
M. Ceretti1, O. Wahyudi2, A. Cousson3, A. Villesuzanne2, M. Meven5, B. Pedersen4, W. Paulus1
1Institut Charles Gerhardt, Université de Montpellier, Montpellier, France
2ICMCB, CNRS-Université de Bordeaux, Pessac, France
3Laboratoire Léon Brillouin, Gif sur Yvette, France
4Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
5Institute of Crystallography, RWTH Aachen at MLZ and Jülich Centre for Neutron Science (JCNS) at MLZ, Garching, Germany
Nd2NiO4+δ and Pr2NiO4+δ are today considered the most promising candidates for mixed electron/ion oxygen conductors at moderate temperatures. In order to investigate their oxygen diffusion pathways, we explored their structure via single crystal neutron diffraction. Large size and high quality single crystals were grown by using an image furnace. While the real structure for both compounds is incommensurate, the scattering density of the respective average structures was reconstructed using the Maximum Entropy Method. Unusually high displacement factors were found for the equatorial and apical oxygen atoms, showing large amplitudes. At 400 °C the anharmonic apical oxygen displacements are strongly enhanced, showing a double well potential and pointing towards the interstitial vacancy sites, creating a quasi continuous shallow energy diffusion pathway between apical and interstitial oxygen sites.
High quality, large size single-crystals of Nd2NiO4+δ (NNO) and Pr2NiO4+δ (PNO) were successfully grown with the help of a mirror furnace, using the floating zone method [1]. The optimized growth parameters allowed large and homogeneous crystals without significant mosaic spread and stacking faults to be obtained. Both compounds crystallize in the K2NiF4 structure type and can accommodate extra oxygen atoms on interstitial sites, the non-stoichiometric region being 0 < δ <0.25. This makes it possible to study the interplay of interstitial and apical oxygen atoms together with structural and lattice dynamic modifications, which are important factors with respect to diffusion mechanisms. In particular, we analysed more accurately oxygen displacement factors in PNO and NNO related to oxygen doping on interstitial sites, which is supposed to induce phonon assisted oxygen diffusion at moderate temperatures. To achieve this, we explored temperature dependent high resolution single crystal neutron diffraction studies combined with data analysis using the Maximum Entropy algorithm (MEM).
Oxygen diffusion pathway
The nuclear structures of the as-grown single crystals were investigated using single crystal neutron diffraction on different neutron 4-circle diffractometers (5C2@LLB at the ORPHEE reactor in Saclay, HEiDi@MLZ and RESI@MLZ at the FRM II reactor in Garching).
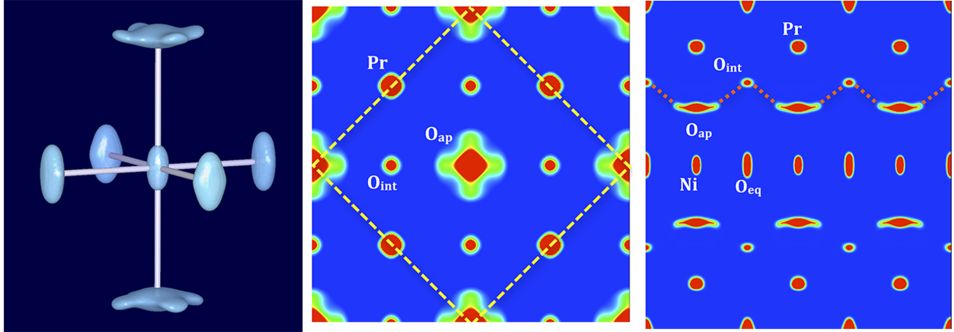
Figure 1: Representation of the nuclear densities of Pr2NiO4.25 obtained from MEM reconstruction of single crystal neutron diffraction data at room temperature. On the left: isosurfaces (density: 2 fm/ Å3) of the NiO6 octahedron; in the middle: 2a × 2a projection of the I4/mmm unit cell (the F-centred cell is outlined by yellow dashed bars) and on the right 3a × c projection of the I-cell. A dashed red line indicates the oxygen diffusion pathways along the [110]-direction in the F-cell (equivalent to [100] of the I-cell), between apical and interstitial sites. © Figure 1: Representation of the nuclear densities of Pr2NiO4.25 obtained from MEM reconstruction of single crystal neutron diffraction data at room temperature. On the left: isosurfaces (density: 2 fm/ Å3) of the NiO6 octahedron; in the middle: 2a × 2a projection of the I4/mmm unit cell (the F-centred cell is outlined by yellow dashed bars) and on the right 3a × c projection of the I-cell. A dashed red line indicates the oxygen diffusion pathways along the [110]-direction in the F-cell (equivalent to [100] of the I-cell), between apical and interstitial sites.
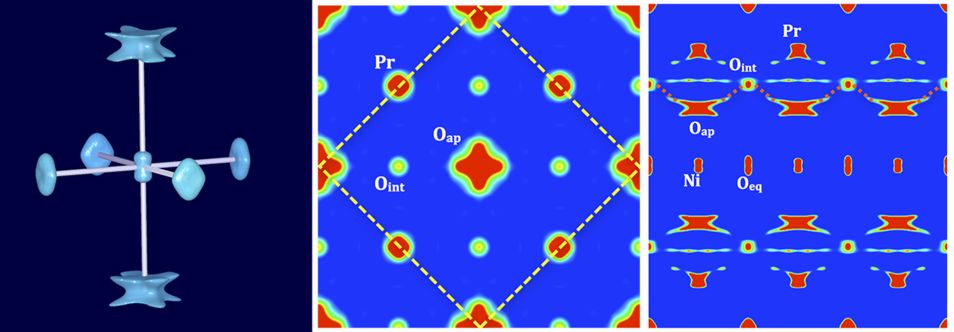
Figure 2: Representation of the nuclear densities of Pr2NiO4.25 obtained from MEM reconstruction of single crystal neutron diffraction data at 400 °C. Left: isosurfaces of the NiO6 octahedron (density: 2 fm/Å3); middle: 2a × 2a projection of the I4/mmm unit cell (the F-centred cell is outlined by yellow dashed bars) and on the right 3a × c of the I-cell. A dashed red line indicates the oxygen diffusion pathways along the [110]-direction in the F-cell (equivalent to [100] of the I-cell), between apical and interstitial sites.
Even though the real structure is quite complex, showing an incommensurate modulation, the refinements were done in the average space group
F4/mmm (equivalent to the standard space group
I4/mmm). The resulting phased Fobs were subsequently used for MEM reconstruction. This approach has the advantage of according equal importance to strong and weak reflections, resulting in a much better defined background, when compared to the classical Fourier-methods. In addition, it yields an anharmonic description of the scattering densities, the only constraint on describing scattering densities being imposed by the symmetry of the used space group.
Neutron single crystal diffraction measurements have been performed in air at RT and at 400 °C [2], just above the incommensurate-commensurate phase transition, occurring at 360 °C.
At RT, the apical oxygen scattering densities correspond to an anisotropic libration mode in the (a,b)-plane and specifically along [110], on an outer perimeter of about 2 Å, with a tilt angle of the NiO6 octahedron of about 25°. It becomes evident that the large amplitudes of the apical oxygen atoms, pointing towards the adjacent interstitial sites, generate a low energy diffusion pathway between these two sites (right part of Fig. 1). The [110] displacements of the apical oxygen atoms correspond to almost half of the diffusion pathway towards the interstitial sites. One may presume these displacements to be in part dynamically activated, leading to significant oxygen mobility already at ambient temperature. The disorder scenario of the apical oxygen atoms is completely modified at 400 °C compared to the room temperature structure, showing a double well potential and strongly increasing the anharmonic displacements of the apical oxygen atoms. As evidenced in Figure 2 (right part), the outer parts of the Oap displacements towards [110] become much more pronounced, pointing to a significant extent towards the interstitial sites, compared to what is found at ambient temperature
Towards a phonon assisted diffusion
mechanism
The anharmonic behaviour concerns the whole Pr2O2 rock salt layer, which can be regarded as dynamically decoupled from an embedding matrix of NiO2 layers. This highlights the important role of lattice dynamics in contributing to the 2D oxygen diffusion mechanism at already moderate temperatures. These studies clearly underline the limitations of the classical approaches to describe these anomalously anharmonic lattice dynamics, when using harmonic potentials. Our results confirm the origin of the low temperature oxygen diffusion mechanism in K2NiF4-type oxides to be related to structural instabilities of the apical oxygen atoms and associated phonon softening, allowing large apical oxygen displacements to dynamically promote oxygen diffusion [3,4].
References:
[1] O. Wahyudi et al., Crystengcomm 17, 6278 (2015).
[2] M. Ceretti et al., J. Mater. Chem. A 3, 21140 (2015).
[3] W. Paulus et al., J. Am. Chem. Soc. 130, 16080 (2008).
[4] A. Villesuzanne et al., J. Solid State Electrochem. 15, 357 (2011).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:


