MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Lithium plating investigated by in situ neutron diffraction
V. Zinth1 , C. von Lüders2, M. Hofmann1, J. Hattendorff3, I. Buchberger3, S. Erhard2, J. Rebelo-Kornmeier1, A. Jossen2, and R. Gilles1
1Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
2Lehrstuhl für Elektrische Energiespeichertechnik, Technische Universität München, München, Germany
3Lehrstuhl für Technische Elektrochemie, Technische Universität München, Garching, Germany
Li plating, the deposition of metallic lithium onto the graphite anode of a Li-ion cell was studied using in situ neutron diffraction at Stress-Spec. Competing with the intercalation of lithium into graphite that takes place during the normal charging process, Li plating results in a lower degree of graphite lithiation during and after charge: After fast charge at -20 °C, neutron diffraction shows there is less highly lithiated LiC6 than after slower charge, although the cell stores the same amount of charge. Li plating uses up to 19 % of the active lithium in the cell. However, Li plating is mostly reversible and the metallic lithium slowly diffuses into the graphite during 20 h rest or is discharged prior to the lithiated graphite during immediate discharge [1].
Lithium and Li-ion batteries
In rechargeable Li-ion batteries, Li ions are transported to the negative electrode (anode) during charge and move back to the positive electrode (cathode) during discharge. The first rechargeable lithium batteries developed in the 1970s used metallic lithium as anode material. Unfortunately, during repeated charging, metallic Li does not deposit in a uniform way on the Li anode, but forms dendrites that can lead to internal short circuits in the cell, in the worst case causing the cell to burn or even explode. Because of these hazards, rechargeable batteries with a Li anode were never commercialized [2].
The breakthrough and commercialization of rechargeable Li-ion batteries – used nowadays in many applications such as smart phones, cameras and laptops – came only in the 1990s with the use of the intercalation materials graphite and LiCoO2. In graphite, Li is reversibly intercalated between the graphite layers while the cell is charged and the problem of dendrite formation is avoided under normal operating conditions. Still, the potential of Li insertion into graphite is close to the potential for the formation of metallic lithium and during fast charge or at low temperatures, where the kinetics of intercalation are too slow, the deposition of metallic lithium on the graphite anode – so called Li plating – can still occur. Possible consequences are dendrite formation, faster capacity loss and cell aging. All these issues should be avoided in a safe and long-life Li-ion cell.
So far, evidence for Li plating has come only from a “bump” in the discharge curve (a so called high-voltage plateau) [3], some microscopic studies with cell designs far from standard commercial cells and ex situ studies, where aged cells were disassembled and metallic or white areas on the electrodes interpreted as the result of Li plating. Here, neutron diffraction offers a promising alternative for investigating Li plating in a commercial cell under operating conditions.
Investigation of Li plating at Stress-Spec
While the metallic lithium cannot be observed directly due to its small quantity and scattering in comparison to the other cell components, its presence is implied by a lower degree of graphite lithiation, since Li ions can either be reduced to metallic lithium or intercalated in the graphite anode. Li intercalation into graphite proceeds via a number of stages from graphite to LiC12 and finally LiC6, both easily identified by neutron diffraction because of their prominent (002) and (001) reflections. At Stress-Spec, the high neutron flux and the area detector make it possible to collect diffraction data on these reflections in about 5 min intervals during cell charge and discharge. Since Li plating is enhanced at low temperatures, experiments were performed at -20°C with the Li-ion battery, a commercial 18650 cell with a graphite anode and a LiNi0.33Mn0.33Co0.33O2 cathode, being placed in a cryostat.
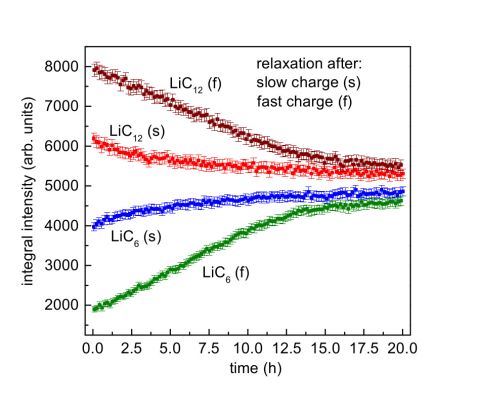
First, the cell was charged very slowly within 30 h and discharged again after a 20 h rest. In a second cycle, the cell was charged within 5 h, again followed by a 20 h rest. Comparison of the diffraction data collected at the end of the slow, 30 h charge and the fast, 5 h charge showed that after the 5 h charge the graphite was lithiated to a lower degree than after the 30 h charge. After the 5 h charge, less LiC6 – the most lithiated form of graphite – was present, and more LiC12 remained. At the same time, the charge put into the cell and extracted from it was almost the same, which means part of the charge must have been stored in the cell in a way different from lithiated graphite: as metallic lithium.
What happens to Li plating after charge?
During a 20 h rest after charge, the LiC6 reflection intensity increases further and the LiC12 reflection intensity decreases (Figure 1). The changes are more pronounced after fast charge. This means the graphite is further lithiated during rest, especially after fast charge, supposedly by the reaction of the plated metallic lithium with the graphite anode and diffusion of Li ions into the graphite particles. This shows a major part of Li plating is reversible and given enough time, the plated lithium may diffuse into the graphite.
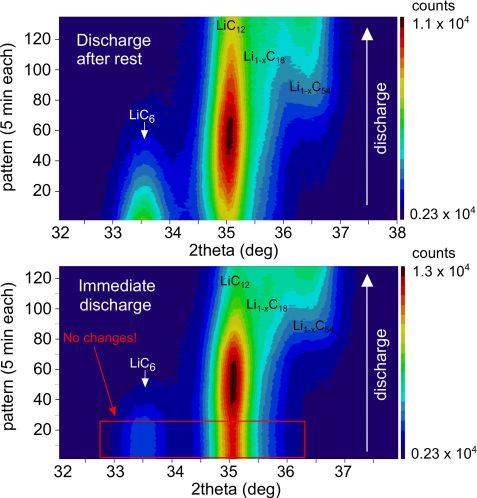
What happens to plated Li during discharge?
During discharge, Li deintercalates from the graphite. LiC6 is transformed first to LiC12 and then to a number of phases with lower lithium content that are found to coexist at low temperatures. However, if the cell is discharged directly after fast charge, at first we see no such changes. As figure 2 shows, there are no changes to the LiC6 and LiC12 reflections, although charge is extracted from the cell and consequently Li ions must move from anode to cathode. This means the metallic lithium is discharged first. Then, after a certain time, the discharge proceeds with the delithiation of the graphite, and the Li extraction from the graphite and LiC6 transformation to LiC12 begins. This coincides with the end of the above mentioned high voltage plateau, also observed in our experiment. From the charge extracted from the cell before the delithiation of graphite begins, we can estimate the percentage of metallic lithium, which is 19 % of the cyclable lithium in the cell.
So far, we have been able to estimate the amount of Li plating in a commercial Li-ion cell and show that most of that plating is reversible and can either diffuse into the graphite anode during a rest period or be discharged prior to the discharge of lithiated graphite. In situ neutron diffraction gives unique insights into the phenomen of Li plating. In the future, a deeper understanding of Li plating may help may provide better means to detect and prevent it.
References:
[1] V. Zinth et al., J. Power Sources 271,152 (2014).
[2] J. B. Goodenough et al., J. Am. Chem. Soc. 135 (4), 1167 (2013).
[3] R. V. Bugga et al., ECS Trans. 25 (36), 241 (2010).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien: