MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Influence of Ibuprofen on phospholipid layers
S. Jaksch1, F. Lipfert1, A. Koutsioubas1, S. Mattauch1, O. Holderer1, O. Ivanova1, S. Hertrich2, S. F. Fischer2, B. Nickel2, H. Frielinghaus1
1 Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
2 Physik-Department, Technische Universität München, and CeNS, Ludwig-Maximilians-Universität München, München, Germany
A basic understanding of biological membranes is of paramount importance as they comprise the very building blocks of life itself. Cells depend on a range of properties of the membrane in order to function. We investigated the influence of ibuprofen on the structure and dynamics of L-α-phosphatidylcholine (SoyPC) membranes using grazing incidence small-angle neutron scattering (GISANS) and neutron reflectometry at the Heinz Maier-Leibnitz Zentrum (MLZ) [1]. We were able to determine that ibuprofen induces a two-step structuring behavior in the SoyPC films, where the structure evolves from a lamellar phase for pure SoyPC, through a superposition of two hexagonal phases to a hexagonal phase at high concentrations. This behavior may be instrumental in explaining the toxic behavior of ibuprofen in long-term application.
Phospholipid membranes as model systems
Phospholipid membranes are used as model systems for the study of biological cell membranes. Aeffner et al. reported stalk formation in such systems [2], [3]. While the relative humidity was used as a stimulus in those studies, we have used ibuprofen. Ibuprofen is a common drug used as a painkiller and for the treatment of cancer [4] and Alzheimer’s [5]. SoyPC is a phospholipid known to form multilayers. Previous studies have reported ibuprofen to be toxic in long-term application, which has been linked to an increased permeability of the cell membrane [6], [7]. We investigated fully hydrated SoyPC membranes deposited on hydrophilic silicon surfaces at 35 °C.
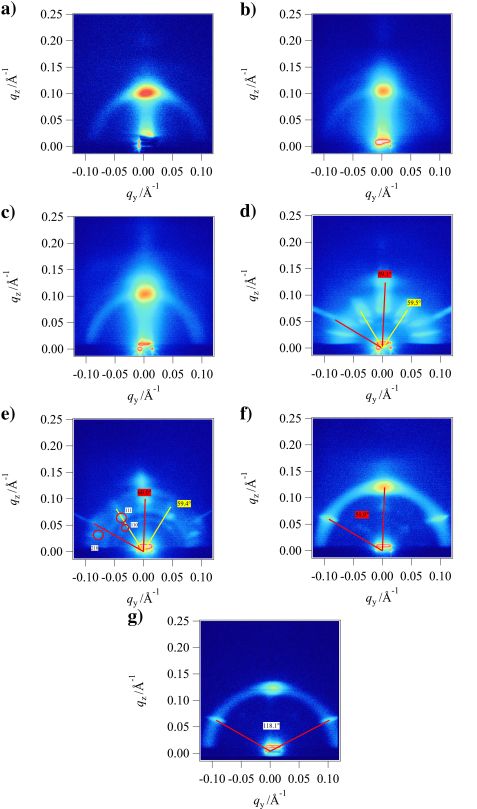
Figure 1: GISANS images at an incident angle of 0.2°. Concentrations of ibuprofen are (a) 0 mol %, (b) 13.6 mol %, (c) 25.0 mol %, (d) 34.5 mol %, (e) 43.1 mol %, (f) 50.2 mol %, and (g) 53.3 mol %. Scattering with a hexagonal symmetry is shown by yellow and red lines; colored labels show the angles between the respective lines. Indexed peaks are shown by red (hexagonal lattice with parallel axis to the surface), yellow (hexagonal lattice with perpendicular axis to the surface), and black (primitive tetragonal lattice) circles, where labels show the indexes.
GISANS – Evolution of the structure
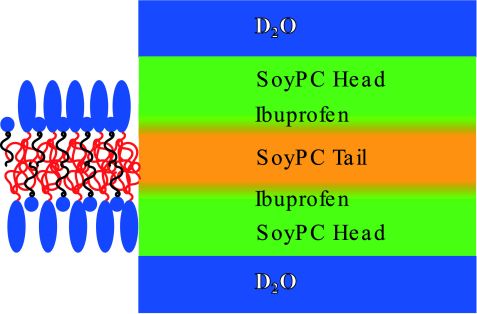
Figure 2: Depiction of the layer model used by the Parratt algorithm. For better visibility, the hydrophobic parts of SoyPC are in red, and those of ibuprofen are in black.
GISANS images of all samples can be seen in Fig. 1. These images show a clear evolution from a lamellar-based scattering to a scattering where several different structures contribute to a hexagonal structure with additional disordered lamellae, as can be seen from the persisting Debye-Scherrer ring.
At low concentrations, one single main maximum from lamellar scattering is visible. While hardly visible at 0 mol %, a Debye-Scherrer ring starts to appear at 13.6 and 25.0 mol %. This can be described as a powder of lamellar regions in the scattering volume.
Hexagonal structures emerge at 34.5 mol %. We find two hexagonal structures. In the 43.1 mol % GISANS image, the non-hexagonal peaks were indexed as the peaks of a primitive tetragonal lattice. Only a single hexagonal lattice is retained at 50.2 mol % and above.
Neutron reflectometry – Intra membrane composition
Reflectometry revealed the layer structure of the system as depicted in Fig. 2. In all systems, about 35 – 40 repetitions of these layers were found.
Another observation from these data is that the modeled roughness of the layers is maximal at 25.0 mol % ibuprofen. We attribute this roughness to a high strain of the membrane that occurs at the onset of ordering.
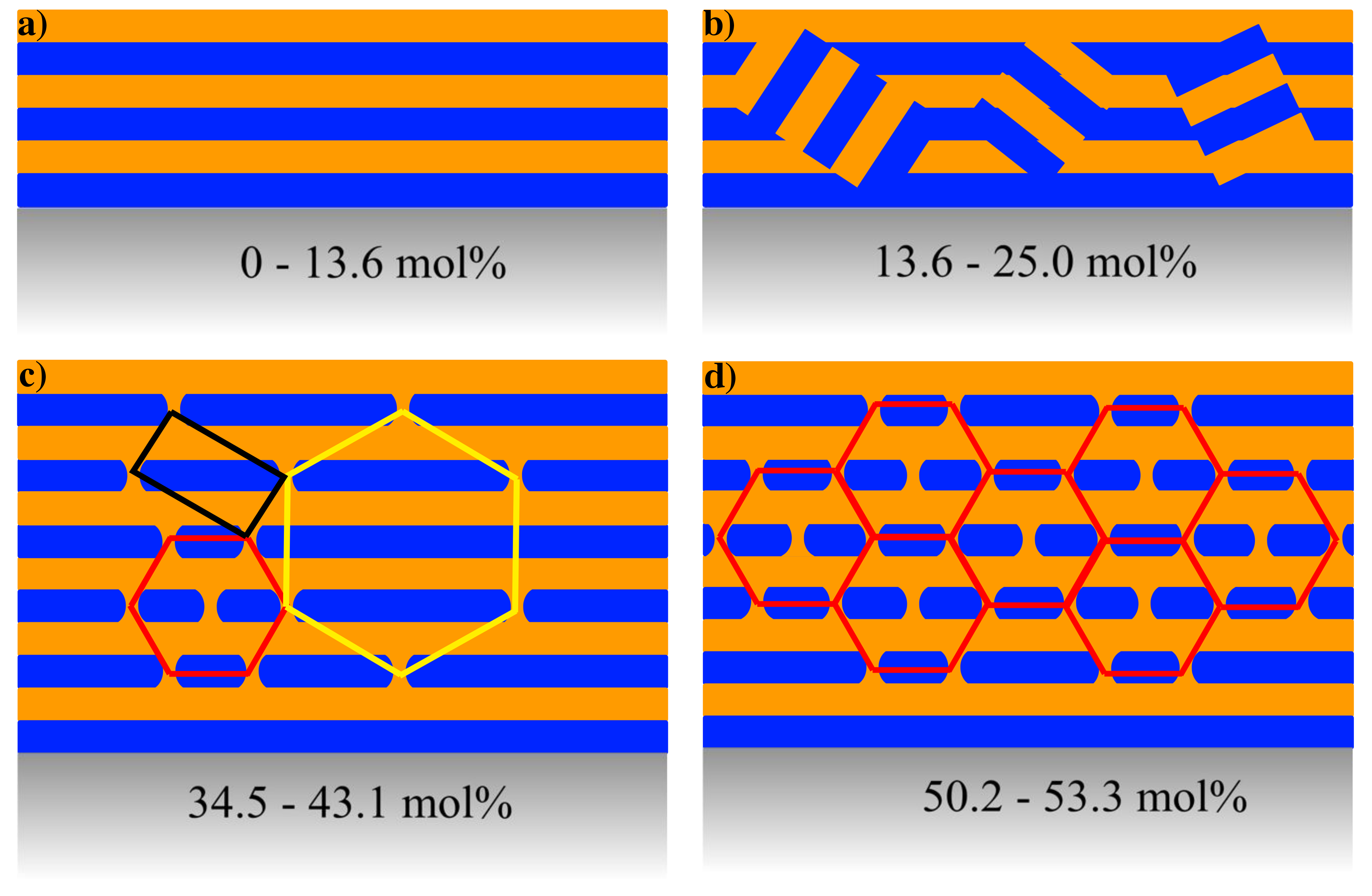
Figure 3: Sketch of the structural evolution of the sample. (a) For low concentrations of ibuprofen, the system is dominated by a lamellar structure while the introduction of more ibuprofen (b) induces disordering of lamellar areas and thus powder scattering of lamellar areas. In an intermediate concentration area (c), there are two different hexagonal lattices that are stabilized against each other by a primitive tetragonal lattice. At very high concentrations (d) only the hexagonal structure with an axis parallel to the substrate is retained. Color coding of the hexagonal structure corresponds to the color coding used in Fig. 1.
Overview and impact on toxicity
An overview of the structures observed is shown in Fig. 3. The introduction of ibuprofen into SoyPC phospholipid films has several effects. First, we observed the emergence of several coexisting lattices, and finally a single hexagonal lattice at 53.3 mol % ibuprofen in the film.
Regarding the toxicity, we have to bear in mind that the ibuprofen concentrations investigated here are beyond any medical applicability. However, it is conceivable that in the case of long-term treatment, where these complications occur, once a nucleation point for this damage is created, the damage starts to grow.
References:
[1] S. Jaksch et al., Phys. Rev. E 91, 022716 (2015).
[2] S. Aeffner et al., Eur. Phys. J. E 30, 205 (2009).
[3] S. Aeffner et al., Proc. Natl. Acad. Sci. U. S. A. 109, E1609 (2012).
[4] M. J. Thun et al.; J. Natl. Cancer Inst. 94, 252 (2002).
[5] S. Weggen et al., Nature 414, 212 (2001).
[6] L. M. Lichtenberger et al., Nat. Med. 1, 154 (1995).
[7] M. B. Boggara et al., J. Phys. Chem. B 114, 8061 (2010).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:


