MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Effect of proteins on calcium mineralization in solutions associated with wastewater desalination
Y. Dahdal1, V. Pipich2, R. Kasher1, Y. Oren1, D. Schwahn3
1Zuckerberg Institute for Water Research, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Israel
2Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
3Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
A serious issue that arises in the reclamation of potable water using membrane technology such as reverse osmosis is that of biofouling and scaling. In particular, the formation of calcium phosphate at the surface of membranes poses a serious problem. In this report, we present SANS studies on the effect of the protein BSA on mineralization in a model salt solution (SSE), simulating the secondary effluent of a wastewater reclamation plant. These studies are important as the microorganisms that exist in wastewater excrete organic molecules such as proteins and polysaccharides. Individual and grafted BSA on gold nanoparticles was exposed to SSE. It is shown that BSA immediately induces stable composite particles in the micrometer scale. The dissolved BSA monomer is much more effective with respect to mineralization as compared to BSA grafted on gold nanoparticles. This is an interesting result as the grafted BSA simulates in some respects a biofilm at the surface of the membranes.
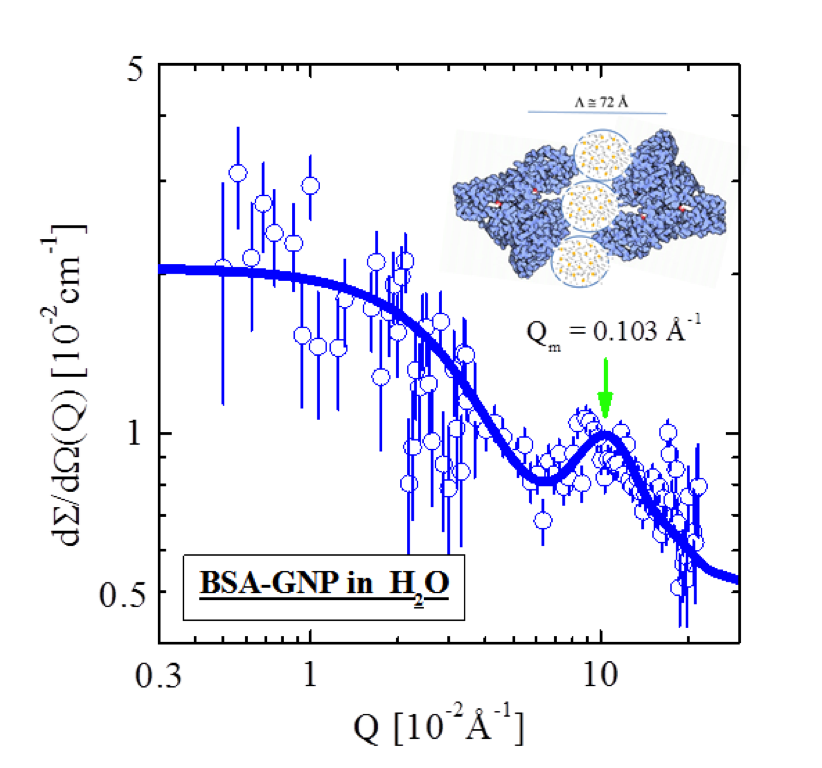
Figure 1: Scattering from BSA-GNP colloids in salt-free H[~2~]O.
Reverse-osmosis (RO) desalination is an attractive technology nowadays for achieving high quality potable water from pretreated domestic wastewaters. One of the main limitations on cost-effective RO desalination is fouling of the membranes, mainly biofouling and scaling by calcium phosphate. The feed for RO treatment, i.e. the solution to be reclaimed as potable water, contains salt molecules as well as biomolecules such as proteins and polysaccharides excreted from microorganisms. These molecules strongly influence the mineralization of calcium phosphate and carbonate, as they emerge from specific interactions between organic and inorganic components widespread in nature and extensively studied under the concept of biomineralization [1,2].
We explored these phenomena using the technique of small-angle neutron scattering (SANS) at KWS-1 and 2 at MLZ [3-5]. The organic molecule bovine serum albumin (BSA) was exposed to a simulated secondary effluent (SSE) in order to study its effect on mineralization. The SSE solution represents a model salt solution simulating a RO desalination concentrate of the Shafdan secondary wastewater reclamation plant in Israel [6]. In these experiments, processes were simulated which occur in the feed at the high pressure side of the RO device during desalination.
Grafted protein BSA dissolved in salt-free water
We studied BSA as a free molecule and as grafted on gold nanoparticles (BSA-GNP) in order to simulate BSA attached as a biofilm at the membrane surface. The molecules were first characterized in salt-free water. Fig. 1 shows scattering from the BSA coated gold nanoparticles (BSA-GNP), which could be analyzed with the form factor of dimers, depicted as a solid line. The dimers are composed of two BSA monomers of 64.5 Å diameter at a distance of around 72 Å and attached by about three GNPs.
Proteins in SSE
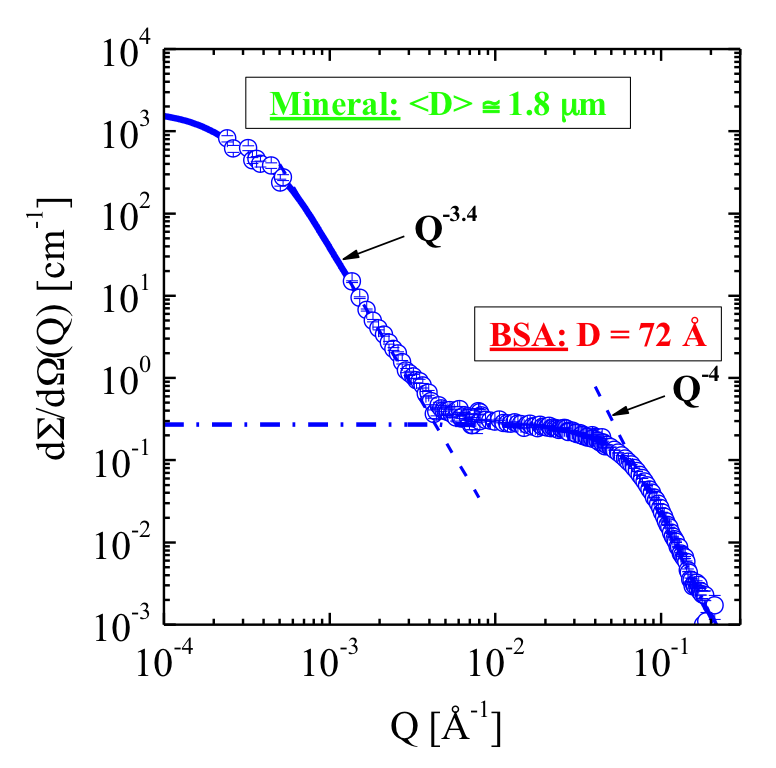
Figure 2a: Scattering immediately after dissolving BSA in SSE. At small and large Q values μm large protein-mineral composite particles and BSA monomers are observed, respectively.
Dissolving BSA in SSE leads to the fast formation of stable colloids in the micrometer scale within a few seconds. Fig. 2a shows a scattering pattern determined immediately after mixing BSA in SSE. Strong scattering due to the formation of micron-sized particles is observed at small Q values, whereas at large Q’s scattering from BSA monomers is visible. Quantitative analysis shows that only ∼20 % of BSA is involved in the mineralization of composites of volume fraction ∼2.3 × 10-4 which are composed of BSA and mineral of ∼34 % and ∼66 %, respectively. Fig. 2b shows BSA-GNPs in salt-free water as well as its effect of initiating a large increase in scattering when exposed to the salts of SSE. Analysis shows that colloidal particles of ∼0.3 μm diameter and volume fraction ∼3 × 10-5 are formed and are composed of ∼18 % protein and ∼81 % mineral. The latter information regarding the composite particles was derived from contrast variation of the SSE-H2O/ SSE-D2O mixture.
Relevance of proteins for the process of RO Desalination
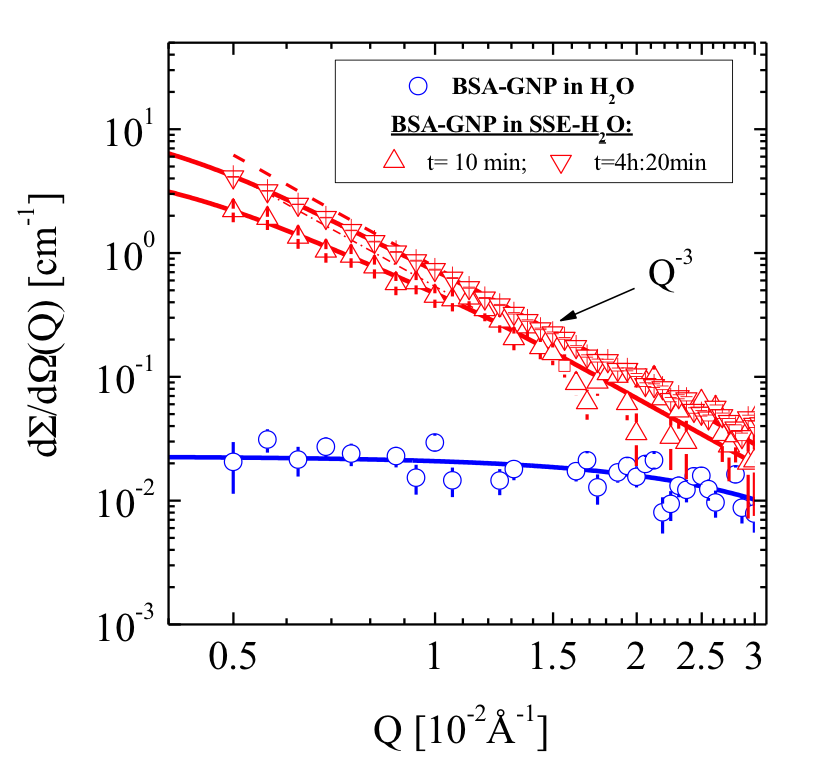
Figure 2b: BSA-GNPs in salt-free water (H[~2~]O) and SSE-H[~2~]O. Mineralization shows strong enhanced scattering.
Modification of BSA is at the root of the main difference in the SANS scattering patterns presented in Fig. 2a and 2b. The apparent similarity of both studies is the fast process of the formation of stable organic-inorganic composites after adding both types of BSA entities to SSE. On the other hand, the BSA dimers with the order of 10-5 volume fraction induced a much smaller amount of mineral than BSA monomers which induced a mineral volume fraction of the order of 10-4. A reasonable explanation might be that mineralization by BSA monomers occurs at any point on the protein monomer’s surface, whereas BSA coated at GNPs adopts a constrained structure so that only part of the interface was exposed to the SSE solution.
Summary
This study unveils a strong interrelation between bio-molecules, represented in the present case by the protein BSA and the formation of calcium minerals, and of calcium phosphate in particular. It has been shown that GNP bound BSA dimers (Fig. 1) are less prone to calcium mineralization in comparison with dissolved BSA monomers. The results obtained from the present model system SSE suggest that removing both the organic macro-molecules and calcium phosphate nanoparticle in tertiary wastewater effluents prior to RO desalination may be highly beneficial. On the other hand, bio-molecules such as BSA are effective coagulants of calcium minerals. This observation might stimulate further research with the aim of finding chemical substances with similar organic functional groups which are sufficiently cheap and effective to be applied as coagulants prior to a RO of nanofiltration (NF) processes.
Acknowledgements
Funding by the Ministry of Science, Culture and Sport (MOST), Israel and the Bundesministerium für Bildung und Forschung (BMBF), Germany (grant no. WT0902), and by the German-Israeli Foundation for Scientific Research and Development (GIF) (grant no. I-101-307.4-2013) is gratefully acknowledged.
References:
[1] S. Mann, Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford (2001).
[2] V. Pipich et al., J. Am. Chem. Soc. 130, 216879 (2008); A. Heiss et al., Biophys. J. 99, 3986 (2010).
[3] V. Pipich et al., Langmuir 29, 7607 (2013).
[4] Y. N. Dahdal et al., Langmuir 30, 15072 (2014).
[5] Y. N. Dahdal et al., Polymer 85, 77 (2016).
[6] Z. Steiner et al., Environ. Sci. Technol. 44, 7937 (2010).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:


