MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Contrast matching SANS reveals structural properties in protein-gold hybrid materials
G. Nyström1, M. P. Fernández-Ronco2,3, S. Bolisetty1, A. Radulescu4, M. Mazzotti2, R. Mezzenga1
1 Laboratory of Food & Soft Materials, ETH Zürich, Zürich, Switzerland
2 Institute of Process Engineering, ETH Zürich, Zürich, Switzerland
3 Laboratory for Advanced Fibers, EMPA, St. Gallen, Switzerland
4 Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
Gold, with more than 5000 years of history, has been of enormous historical and cultural importance across civilizations. Its beauty and malleability made it highly desirable for ornamentation, jewelry and for use as a currency. The high value and price of gold has lasted to this day and creates a demand for the efficient utilization of gold in new lightweight materials. We used gold in the form of microcrystals and nanoparticles combined with protein fibrils to develop gold aerogels – a new class of ultra- light gold materials. By changing the colloidal gold shape, size and concentration we were able to tune the optical properties and final gold composition to reach contents above 20 karat equivalent, yet at densities ~103 lighter than any equivalent gold alloys. In this study, we show how contrast matching small-angle neutron scattering (SANS) has provided important structural information about these materials.
Amyloid fibrils as templates for protein-gold aerogels

Figure 1: Photograph of protein gold microcrystal aerogel.
Traditional silica or metal oxide aerogels are inherently brittle. One approach to improving the mechanical flexibility and, thereby, expanding the aerogel application range is to use organic colloidal precursors such as carbon nanotubes [1], graphene [2] or nanocellulose [3]. While the results reported previously are promising, each system has limitations. These limitations are related in particular to the colloidal stability of the precursor solutions and the flexibility in terms of the nature and maximum loading of additional inorganic particles in the final aerogels.
We recently proposed a new class of aerogel materials based on protein colloids, so called amyloid fibrils [4]. These fibrils, formed by the self-assembly of β-lactoglobulin in water, carry functional groups, [5] that can act as natural reduction sites for inorganic particles. The fibrils also serve the dual purpose of keeping the organic-inorganic hybrid dispersion stable even at high inorganic particle concentrations. To produce gold protein hybrid aerogels, gold was added in salt form (HAuCl4) to the amyloid fibrils. Following either incubation at pH 2 and 70 °C or chemical reduction using NaBH4, gold microcrystals or gold nanoparticles stabilized with amyloid fibrils were formed. The addition of 150 mM NaCl transferred the dispersions to free standing hydrogels that were subsequently solvent-exchanged to ethanol and non-destructively dried to aerogels using supercritical CO2.
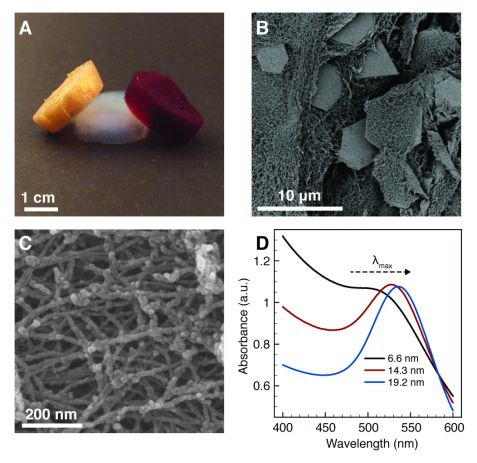
Figure 2: Photograph (A), electron micrographs (B-C) and UV/Vis characterization (D) of the protein and protein-gold hybrid aerogels.
The aerogels obtained had very low densities (2.9 – 30.8 mg/cm3) and high specific N2 surface areas (325 m2/g). By changing the size, shape and mass fraction of the gold particles, the optical properties of hybrid aerogels could be tuned (Fig. 2). Aerogels containing plate-like gold microcrystals (Fig. 2B) showed a traditional golden color, whereas the spherical gold nanoparticles (Fig. 2C) gave the aerogels a characteristic ruby red appearance that was tunable by particle size (Fig. 2D). The visual appearance of the gold microcrystal aerogels is almost identical to the appearance of traditional solid gold metal (Fig. 1). This is remarkable for a material that consists of more than 98 vol% of air.
The aerogel material presented may be used in bio-sensing, radiation protection, food decoration, as a replacement for traditional gold in jewelry or as components of artworks. We have explored the gold microcrystal aerogel as a pressure sensor and the gold nanoparticle aerogel as a substrate for heterogeneous catalysis [4]. The aerogels can also be crosslinked with glutaraldehyde vapor to render them wet-stable. This further broadens their application range and allows for a structural characterization of the samples under wet conditions, specifically using contrast matching SANS.
Contrast matching SANS on cross-linked protein-gold aerogels
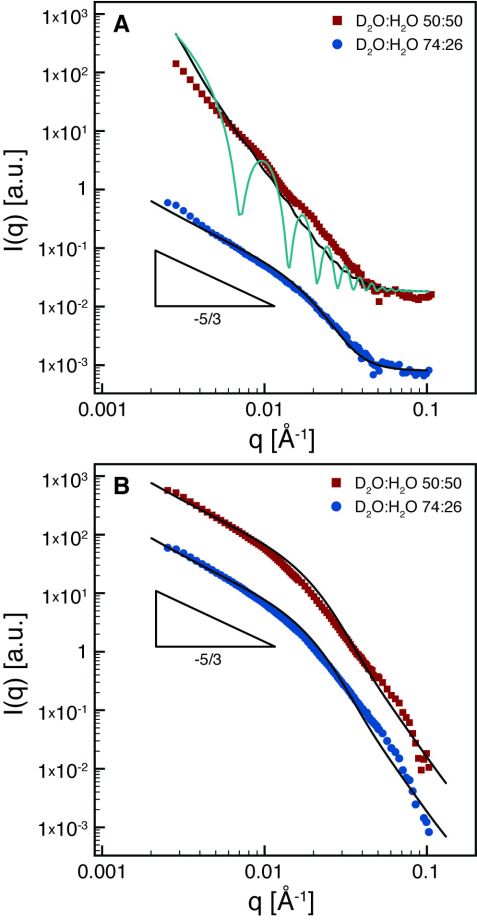
Figure 3: SANS measurements of the protein gold crystal (A) and protein gold nanoparticle (B) aerogels at solvent matching conditions for protein fibrils (red squares) and solvent matching conditions for gold (blue circles). The scattering data have been offset for clarity.
Contrast matching SANS was used to selectively investigate the distribution of amyloid fibrils and gold particles within the aerogels (Fig. 3). At the contrast matching conditions for gold (D2O:H2O = 74:26 vol%), we found that the scattered intensities decay with a slope of 5/3 for both types of gold particles (Fig. 3 blue circles). This slope is characteristic of the fractal exponent of a self-avoiding random walk and highlights the semi-flexible nature of the amyloid fibrils. When the solvent is matched to the amyloid fibrils (D2O:H2O = 50:50 vol%), different shapes of the scattering curves are recovered, reflecting the different form factors of the gold particles (Fig. 3 red squares). For the gold nanoparticle aerogels, (Fig. 3B) the scattering closely resembles the scattering from the amyloid fibrils. This indicates that the gold nanoparticles are uniformly coated on the amyloid fibrils, in agreement with electron microscopy (Fig. 2C). For the gold microcrystals, a distinctly different scattering profile is found with a higher slope (~3). This reveals the bulk characteristic of the gold microcrystals (lateral dimension in the micrometer scale, Fig. 2B) for q corresponding to length scales of 15 – 200 nm. The continuous lines in Fig. 3 are fitted curves using the form factors for polymers with excluded volume [6] (fibrils) and infinitely large disks with polydisperse (black line) or monodisperse (green line) thickness (gold microcrystals), respectively, showing good agreement between scattering profiles and the expected shapes of the organic and inorganic components. The slight upturn in the form factors of the large disks at low q in Fig. 3A is attributed to extra edges present in the gold single crystals (of fractal dimensionality 1), a feature missing in the form factor of disks.
In conclusion, these results provide the structure and conformation of the protein and gold particles within the aerogel and show the power of contrast matching SANS to reveal decoupled structural information from organic-inorganic hybrid materials.
References:
[1] M. B. Bryning et al., Adv. Mater. 19, 661 (2007).
[2] H. Hu et al., Adv. Mater. 25, 2219 (2013).
[3] Y. Kobayashi et al., Angew. Chem. Int. Ed. 53, 10394 (2014).
[4] G. Nyström et al., Adv. Mater. 28, 472 (2016).
[5] S. Brownlow et al., Structure 5, 481 (1997).
[6] J. S. Pedersen et al., Macromolecules 29, 7602 (1996).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:


