MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:

MLZ
Lichtenbergstr.1
85748 Garching
Biomimetic multifunctional magnetite/gel composites
B.-H. Wu1,2, M. Siglreitmeier2, H. Cölfen2, D. Schwahn3 ,V. Pipich1
1Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
2Department of Chemistry, Physical Chemistry, University of Konstanz, Konstanz, Germany
3Heinz Maier-Leibnitz Zentrum (MLZ), Technische Universität München, Garching, Germany
Nature provides many archetypes of highly ordered systems, and some of these biomaterials are known for their remarkable properties. An amazing example is the teeth of chitons (Fig. 1A-D) consisting of a magnetite/protein-polysaccharide hybrid shell. The fully mineralized teeth display remarkable properties such as outstanding fracture toughness, wear resistance and the highest reported hardness in biominerals [1]. Another intriguing biomaterial is Nacre (Fig. 1E-H), which consists of highly oriented aragonitic crystals and an organic matrix showing striking mechanical properties. Inspired by nature’s fabrication strategies, we attempted to explore the use of biomolecules in the study of the mineralization of materials by combining the properties of Chiton teeth and Nacre. The mechanism of bio-inspired magnetite mineralization and the organic-inorganic hybrid structures were studied using SANS.
In the present work, we investigated an in situ mineralization process of magnetic nanoparticles inside the gelatin-chitin (the nacre organic matrix) composite with controllable mineral content varying from
20 wt % up to 70 wt % [2,3]. We were able to show that, with increasing mineral content, the gelatin hydrogel structure as well mechanical and magnetic properties are changed. We explored the role of gelatin hydrogels during bio-inspired magnetite mineralization by using small (SANS) and very small (VSANS) angle neutron scattering at the KWS-1 and KWS-3 diffractometers. The scattering contrast of the aqueous solution was adjusted through the D2O content in order to determine the structure of the organic and inorganic components separately.
Magnetite/gelatin composites
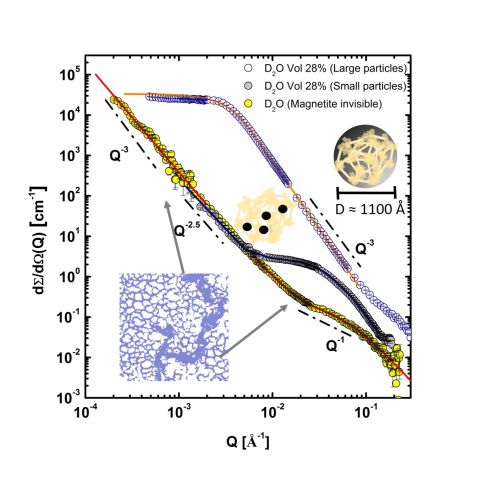
Figure 2: SANS-VSANS scattering profiles present small magnetite particles in the gelatin matrix (obtained by the coprecipitation method) in two contrasts correspondingly: in pure D2O (magnetite is matched, circles with yellow filling) and 28 vol% D2O (matrix is matched, circles with grey filling). The scattering profile of the gelatin-magnetite composite obtained by the partial-oxidation method in gelatin-matching contrast is plotted by symbols with white filling.
Fig. 2 displays three SANS-VSANS scattering patterns of composites dissolved in pure and in 28 vol% D2O, matching the scattering of magnetite and gelatin, respectively. Gelatin hydrogel shows a spatially confined cavity structure appearing as homogeneously distributed cages throughout the gel matrix, which allows small magnetite particles to be formed. The interaction between ions and the network is another key parameter in controlling the mineralization mechanism. Strong interaction between ferric ions and gelatin molecules leads to an inhomogeneous mineralization and a ‘particle in matrix’ structure, while weak interaction between ferrous ions and the gelatin matrix causes a homogenous-like mineralization and a ‘matrix in particle’ structure.
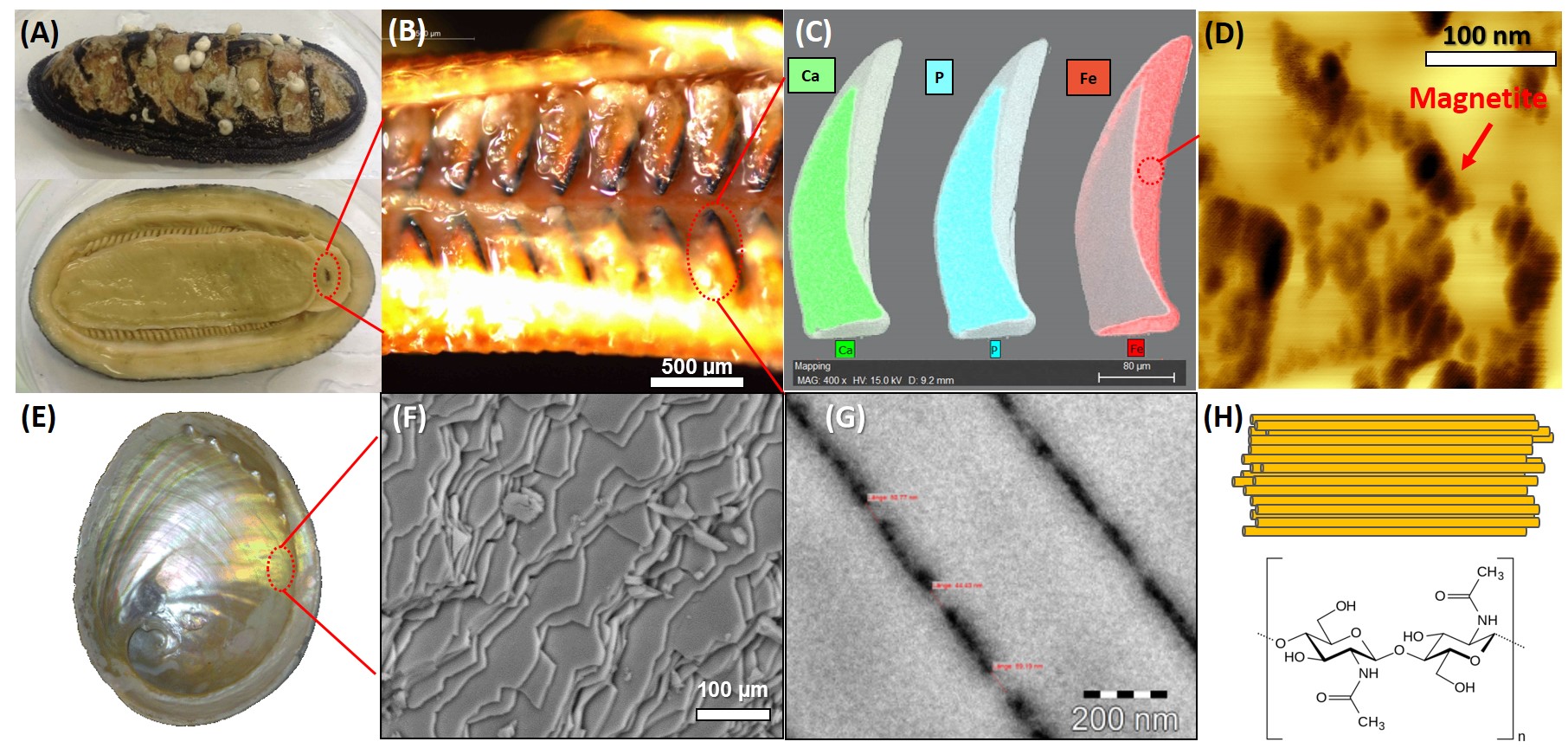
Figure 1: Hierarchical structures of Chiton teeth and Nacre: (A) Chiton; (B) the Chiton radula teeth; (C) SEM EDX results of the teeth; (D) AFM result on the tooth mineral shell; (E) Nacre; (F) Nacre tablet structure (SEM); (G) TEM result on the Nacre mineral bridges structure; (H) the α-chitin texture and the chemical structure.
Multifunctional layered magnetic composites
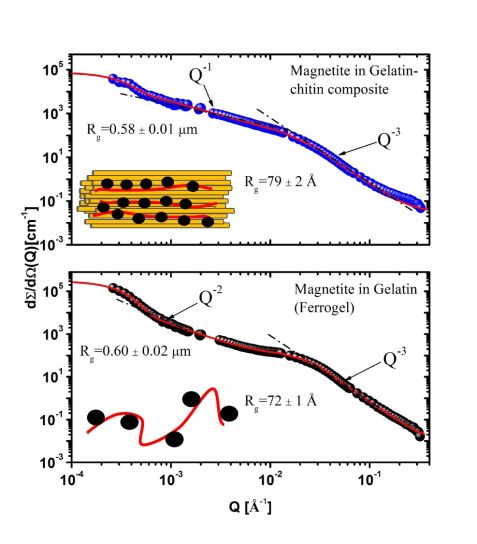
Figure 3: SANS-VSANS scattering patterns of samples measured in the matrix-matching contrast and mineralized by the coprecipitation method: (top graph) corresponds to a hybrid matrix when a demineralized Nacre layer was filled by gelatin, (bottom graph) corresponds to a “pure” gelatin matrix.
The introduction of a nacre organic matrix with chitin fibrous texture as a second organic matrix allows one to influence the mineral organization due to structure spatial constraints. Fig. 3 displays two SANS-VSANS scattering patterns of magnetite in a chitin-gelatin composite (top) and in a gelatin matrix (bottom) dissolved in 28 vol% D2O (magnetite visible). The magnetite-gelatin-chitin sample shows a power law of Q-1 in the low Q-regime, approximately valid for linear structures, thereby indicating rod-like particles or stretched chains of particles. The scattering of magnetite in the gelatin matrix (ferrogel) in the low Q-range shows characteristic behavior of chain-like clusters having a Q-2 power law. Thus, the chitin fiber-like structure of nacre organic matrices supports the formation of linear aligned magnetite nanoparticles (pearl necklace-like, with power law of Q-1), whereas branch- like arrangement of nanoparticles (power law of Q-2) are formed in gelatin gel matrices.
These structural and mineralization mechanisms are compared with the morphology of biological samples such as Chiton teeth, a comparison which will help to optimize the material for improved mechanical performance.
References:
[1] L. M. Gordon, Nature 469, 194 (2011).
[2] M. Helminger et al., Adv. Funct. Mater. 24, 3187 (2014).
[3] M. Siglreitmeier et al., Beilstein J. Nanotechnol. 6, 134 (2015).
MLZ ist eine Kooperation aus:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ ist Mitglied in:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ in den sozialen Medien:


