MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
27.10.2021
What happens to mRNA therapeutics in the body
For the second time, researchers from the Heinz Maier-Leibnitz Centre (MLZ), the global biopharmaceutical company AstraZeneca and Malmö University collaborated to advance the understanding of mRNA therapeutics. This time, their focus was on a specific protein and the question of what happens to the mRNA once it is administered into the blood.
Messenger RNA (mRNA) is the blueprint for the production of proteins that a cell can read and implement. Researchers want to make use of this principle in the form of mRNA therapeutics, with patients being administered mRNA that specifically contains the blueprint for the required proteins. It has many potential applications, from cancer to chronic diseases such as heart failure.
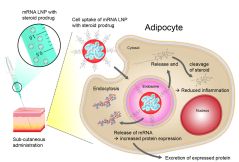
In collaboration with Dr. Aurel Radulescu, instrument scientist at the small angle scattering facility KWS-2 of Forschungszentrum Jülich, scientists from AstraZeneca have already published two papers on mRNA therapeutics research. After the structural characterisation of lipid nanoparticles (LNPs), which serve as packaging and transport medium for mRNA during administration, they investigated in a second step how the tolerance can be improved with subcutaneous application by adding anti-inflammatory agents. This opens up the possibility for patients to self-administer their medication.
The mRNA (green spiral) is packaged in a lipid nanoparticle. Researchers used neutrons to visualize the different structural components of the particle. © Reiner Müller / ACS Nano 2021, 15, 6709-6722
Test under real conditions
For their current work, the research team, led by Dr. Federica Sebastiani at the Department of Biomedical Science, Malmö University, brought in additional support from the University of Malmö. Together they investigated how the mRNA-containing lipid nanoparticles (mRNA-LNPs) bind to proteins in a patient’s blood in a model system, how they are transported to the right place and how they release the mRNA there. The focus was set on apolipoprotein E as the binding partner.
“What is special about this work is that we no longer studied the LNPs in an ideal system, without any interfering factors, but in a real system,” explains Dr. Aurel Radulescu. The environment this time more closely resembles that in the human body.
Neutrons make interfering lipids invisible
To investigate the exact structure of the mRNA-LNPs and the distribution of the individual components in the complex, the scientists use the small-angle scattering system KWS-2. “For the structural analysis, neutrons offer us a unique investigation method,” says Radulescu.
The LNPs consist of three different fat molecules in different proportions. They all have different scattering properties. By deliberately setting the scattering of individual fats to zero by exchanging hydrogen atoms with heavier deuterium atoms, the researchers made them invisible. They thus saw exactly where the individual molecules were located in the structure.
The researchers found that the structure of the mRNA-LNPs changes significantly as soon as they bind to apolipoprotein E in the blood. This leads to a redistribution of the lipid molecules on the surface and in the nucleus, which in turn influences the properties of the LNPs, for example how easily they can release the bound mRNA again.
More precise drug design possible
The results of the research team have the potential to optimize the development of mRNA therapeutics. For example, it is now known that some of the LNPs are initially bound tightly in the liver. Knowing what proportion of the mRNA eventually reaches the target cells of the therapy will make the fate of the mRNA-LNPs in the body clearer. In this way, the researchers have gained an even better understanding of how mRNA therapeutics work.
Original publication:
F. Sebastiani, M. Yanez Arteta, M. Lerche, L. Porcar, C. Lang, R.A. Bragg, C.S. Elmore, V.R. Krishnamurthy, R.A. Russell, T. Darwish, H. Pichler, S. Waldie, M. Moulin, M. Haertlein, V.T. Forsyth, L. Lindfors, M. Cárdenas, Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles, ACS Nano 15(4)(2021), DOI: 10.1021/acsnano.0c10064
Related News
-
11.03.2021
Neutrons for better mRNA vaccines
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: