MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
11.03.2021
Neutrons for better mRNA vaccines

Dr. Aurel Radulescu at the instrument KWS-2 in the neutron guide hall of the Research Neutron Source Heinz Maier-Leibnitz (FRM II) in Garching. © Wenzel Schürmann / TUM
BioNTech, the Mainz-based biotechnology company which together with the US pharmaceutical company Pfizer developed the first Covid-19 vaccine approved in the EU, is also working with the Jülich Centre for Neutron Science (JCNS) on vaccine development. Researcher used the instrument KWS-2 operated by the JCNS at the Heinz Maier-Leibnitz Zentrum.
In 2020 for instance, the researchers, together with the Johannes Gutenberg University Mainz and other partners, published a study on how the efficiency of inserting mRNA into human cells can be improved by optimised packaging. In 2019, the researchers, again in collaboration with their scientific partners, published the results of structural investigations of a model system for packaging mRNA.
mRNA vaccines are a new class of vaccines based on the use of messenger RNA. mRNA serves as a blueprint for the synthesis of protein molecules in human cells. It translates the blueprints stored in the genetic code, the DNA, for the molecular machinery of cells, which connects the building blocks of proteins, the amino acids, to complex molecules using the blueprint.
BioNTech’s Corona vaccine, for example, contains mRNA that encodes the blueprint of the so-called spike protein. The virus uses this to dock onto human cells. The vaccinated cells produce the encoded protein for a short time until the mRNA is naturally degraded and released, thus training the immune system to recognize the foreign protein.
Vaccine against cancer?
It is likely that the method is not only suitable for vaccinations against infectious diseases such as Covid-19. Since, in principle, blueprints for a wide variety of proteins can be introduced into the body this way, it is also hoped that immune reactions against certain types of cancer, for example, can be triggered in this way.
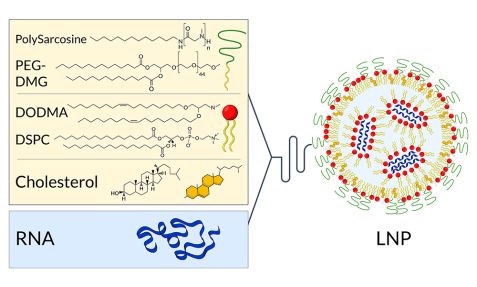
Successfully introducing messenger RNA into cells in the body, known as transfection, is challenging but crucial for the vaccine’s effectiveness. Pure mRNA would quickly be degraded by enzymes found throughout the tissue before it could be absorbed by cells. To protect the precious cargo from damage, it is usually packed in tiny particles known as nanoparticles. These consist of molecules that resemble those of the cell membrane and can therefore fuse with them and deliver the mRNA to the cell interior.
Neutrons test packing material of the mRNA
The researchers used neutron scattering at KWS-2 to investigate various new approaches for packaging and delivering mRNA. Among other things, the investigations showed that the efficiency of transfection can be increased by using the right combination of materials to build the nanoparticles. Suitably constructed hybrid nanoparticles containing both lipids and polymers channel their cargo into the cells better than pure lipid or pure polymer nanoparticles, the scientists reported in 2020 in the scientific journal “Cells”.
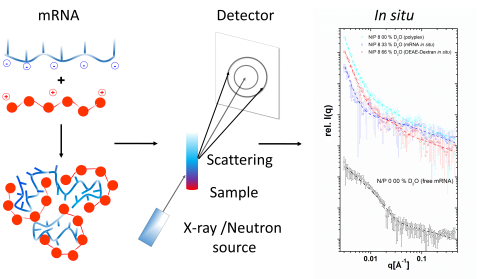
Results of the mRNA (in blue)/DEAE-dextran (in red) particles measured on the KWS-2 instrument (individual components and fully “visible” particles). © Dr. Christian Siewert, Johannes Gutenberg University Mainz
“Structural analyses on the Jülich neutron small-angle scattering instrument KWS-2, among others, showed that the highest transfection efficiency can be achieved using particle types that have a heterogeneous internal organization in which ordered and less ordered regions alternate in a characteristic way,” reports Dr Aurel Radulescu, Instrument Scientist at JCNS.
High neutron flux is an advantage
In order to accurately characterize these particles, the researchers exchanged hydrogen atoms of individual components for heavy hydrogen. Neutrons can distinguish between the two isotopes and thus identify which hydrogen atoms belong to which molecule. “Such experiments require a high neutron flux on the sample, which is the main advantage of the KWS-2 instrument, together with a wide momentum transfer range that allows it to cover a broad length scale in real space.”
The quantity ratio between mRNA and the envelope material also plays a role in the efficiency of transfection: in 2019, the scientists reported in the journal “Biomaterials” on the high-molecular carbohydrate dextran as a “packaging material”. These studies, also undertaken on the KWS-2 instrument among others, revealed a systematic relationship between the quantity ratio of dextran and mRNA and the efficiency of transfection as well as associated structural differences of the nanoparticles.
The use of these model systems and the systematic variation of basic processing steps make it possible to identify correlations between structural properties, biological activity and the production procedure. This understanding supports the further development of complex customised RNA therapeutics and vaccines.
Original text: Angela Wenzik / JCNS
- Information on BioNTech and their mRNA therapeutics
- Original text by Jülich Centre for Neutron Science
Original publications:
Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA; Christian D. Siewert, Heinrich Haas, Vera Cornet, Sara S. Nogueira, Thomas Nawroth, Lukas Uebbing, Antje Ziller, Jozef Al-Gousous, Aurel Radulescu, Martin A. Schroer, Clement E. Blanchet, Dmitri I. Svergun, Markus P. Radsak, Ugur Sahin and Peter Langguth; „Cells“, 2020; DOI: 10.3390/cells9092034
Investigation of charge ratio variation in mRNA – DEAE-dextran polyplex delivery systems; C.Siewert, H.Haas, T.Nawroth, A.Ziller, S.S.Nogueira, M.A.Schroer, C.E.Blanchet, D.I.Sverg, A. Radulescu, F.Bates, Y.Huesemann, M.P.Radsak, U.Sahin, P.Langguth; “Biomaterials”, 2019; DOI: 10.1016/j.biomaterials.2018.10.020
Contact:
Dr. Aurel Radulescu
Jülich Centre for Neutron Science (JCNS-FRM-II)
Forschungszentrum Jülich
Tel. +49 89 289-10712
Email: a.radulescu@fz-juelich.de
Press contact:
Angela Wenzik
Science Journalist
Forschungszentrum Jülich
Tel. +49 2461 61-6048
Email: a.wenzik@fz-juelich.de
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: