MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
16.10.2020
The magic is in the details: Luminous protein decoded more precisely

Scientists of the Heinz Maier-Leibnitz Zentrum have investigated the crystal of the luminescent protein at the instrument BioDiff. © Dr. Andreas Ostermann, FRM II / TUM
The green fluorescent protein GFP is of immense importance as a luminous marker in the cell. At the Heinz Maier-Leibnitz Center (MLZ), Japanese researchers investigated the position of hydrogen atoms in the coloring components of the protein, which enables the development of new marker proteins.
Fluorescent proteins are an important tool in biology, in particular because researchers can link them with other proteins. The green fluorescent protein (GFP) is the best-known fluorescent molecule. When it is irradiated with blue light, it glows green. GFP thus acts as a marker for the molecule with which it is coupled. This enables researchers to directly observe the spatial and temporal distribution of proteins in the living organism: They simply follow the green light under the microscope.
Japanese scientists led by Dr. Motoyasu Adachi from the National Institutes for Quantum and Radiological Science and Technology in Ibaraki, using the MLZ’s BioDiff instrument, succeeded in deciphering the structure of the fluorescent protein at crucial points with greater precision. They investigated the presence and absence of hydrogen atoms (protons) in a mutant of the GFP, called EGFPq. This version has specific mutations at several sites that enhance the fluorescence and stability of the protein. The researcher’s new findings can be applied to the design of future marker proteins.
Neutrons reveal the hydrogen bond networks visible in the chromophore
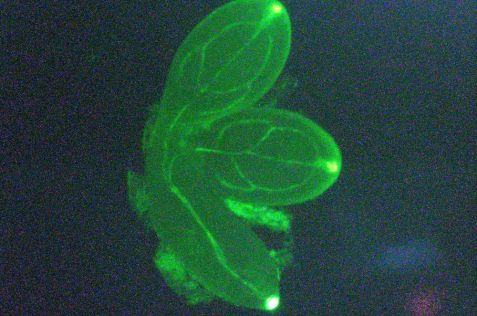
The green fluorescent protein is used as a marker in genetic engineering, as here in the cotyledons of the thale cress Arabidopsis thaliana, to show where a certain protein is produced in the plant. © FRM II / TUM
The BioDiff instrument at the MLZ is designed to determine the structure of biological macromolecules such as proteins. Scientists can use it primarily to investigate the position of the particularly light hydrogen atoms, which is only possible in exceptional cases using other common methods such as X-ray structure analysis.
“One advantage of the monochromatic single crystal diffractometer BioDiff is the high resolution we can achieve,” explains Dr. Andreas Ostermann from the Technical University of Munich. He supported the Japanese scientists in their research efforts for this project, together with his colleague Dr. Tobias Schrader, an instrument scientist at BioDiff affiliated with Forschungszentrum Jülich. “With the data set now measured at a resolution of 1.45 Ångströms, ten millionths of a millimeter, we are among the five best data sets in neutron diffraction for proteins with more than 100 amino acids”. Using neutron crystallography at BioDiff, the researchers were able to analyze the characteristic protonation states of the GFP chromophore and the surrounding key structures.
Even more precise than measurements with X-rays
Using neutron diffraction, the scientists determined the position of the protons in the active center of GFP more precisely than ever before. They found minimal differences in the bond lengths between the hydrogen atoms and the oxygen atom of a central water molecule in the active center. “So the water is a bit distorted,” Ostermann explains. “Our Japanese colleagues presume that this distortion is possibly caused by an unusual electrostatic potential at this site.”
The most exciting distortion is in the building block called “His148”: This structure should be planar – but it is not. This deviation has not been detected with X-rays so far.
The results could contribute to the design of further customized marker proteins. This is often done based on quantum chemical calculations. Protein designers use quantum chemical modeling programs for this purpose. They now can be checked with the new high-resolution data sets and improved if necessary.
GFP: The ‘jack-of-all-trades’ in biology
GFP is of great importance in biology – it is not a surprise that the discoverers were awarded the Nobel Prize in Chemistry in 2008. Today the use of GFP as a marker for other proteins is a standard method in cell biology. Currently, researchers are using GFP in several studies to find out which cells are affected by the SARS-Cov-2 virus in order to better understand the Covid-19 disease.
Original publication:
Shibazaki, C., Shimizu, R., Kagotani, Y., Ostermann, A., Schrader, T. E., & Adachi, M. (2019). Direct observation of the protonation states in the mutant green fluorescent protein. The Journal of Physical Chemistry Letters, 11(2), 492-496. DOI: 10.1021/acs.jpclett.9b03252
Related News
-
30.11.2016
Hydrogen atom hidden and sought
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: