MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
27.01.2022
Understanding the structure of fuel cell membranes

The researchers of the National Institutes for Quantum Science and Technology (QST) during their visit to perform measurements at the MLZ. © Yue Zhao / National Institutes for Quantum Science and Technology (QST), Japan.
Polymer electrolyte fuel cells (PEMFC) can be found, for example, in hydrogen cars and are therefore important in the transition to green energy. The core element of the cells is a proton exchange membrane. Researchers at the Heinz Maier-Leibnitz Zentrum (MLZ) in Garching have now investigated its complex structure in greater detail than ever before with the help of neutrons.
PEMFCs have been developed and in use since the 1960s. The proton exchange membrane (PEM for short) of the PEMFC) is a very thin plastic membrane. It separates the two reaction partners, hydrogen and oxygen, from each other, and allows when hydrated only positively charged electrical particles, the protons, to pass through water-filled channels. Both sides of the membrane are coated with catalytically active electrodes. On one side, hydrogen molecules are oxidised to two protons each by the release of two electrons. These protons then cross the membrane with the help of the channels. On the other side of the membrane, electrons, which previously were able to perform electrical work in an external circuit, reduce oxygen. Together with the protons, water is then formed.
How does the proton exchange membrane work?
Currently, perfluorosulfonic acid polymers such as Nafion are technically the leading materials for PEMs, as they show good mechanical and chemical stability at moderate operating temperatures while simultaneously demonstrating high proton conductivity over a wide range of relative humidity conditions. These properties, according to the latest research, result from the membranes’ structure being made up of nanometre-sized domains, each of which is either hydrophilic or hydrophobic. However, to gain an understanding of the mechanism by which ions are transported through the material and thus learn how to produce more efficient materials for different temperatures and humidity conditions, a complete picture of the microstructure of Nafion was still lacking, despite long-standing, widespread use of the material including intensive studies conducted with X-ray and small-angle neutron scattering, among other techniques.
Neutrons make structures visible
Researchers from the National Institutes for Quantum Science and Technology (QST) in Japan, with the support of Jülich physicist Dr. Aurel Radulescu, have now investigated the microstructure of hydrated Nafion and the water-filled ion channels in more detail for the first time with the help of a special method of contrast variation neutron small-angle scattering on the KWS-2 research instrument operated by Forschungszentrum Jülich at the MLZ. “Neutron scattering is fundamentally an excellent way of making the structures of soft materials visible, unlike electron microscopy, for example, which quickly destroys soft samples,” explains Radulescu. “In addition, neutrons can be used to study soft materials under real conditions, with the option of varying technically relevant sample parameters, such as temperature and moisture content, in a controlled manner.”
Scientists use trick: deuterium instead of hydrogen
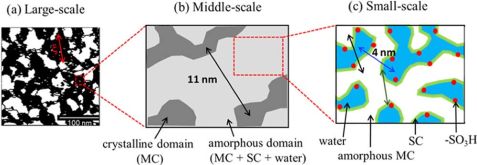
Schematic representation of the self-similar sponge structure of the main chain, side chain and water regions in the Nafion membrane completely covered with water in (left) large scale, (centre) medium scale and (right) small scale. © Yue Zhao, National Institutes for Quantum Science and Technology (QST), Japan. Reprinted with permission from Macromolecules 2021, 54, 9, 4128–4135. Copyright 2021 American Chemical Society.
Unlike a microscope, however, neutron scattering images depict structures indirectly and as a whole entity, so additional steps were necessary to obtain detailed structural information. “We can think of a forest as an analogy,” explains the neutron specialist. “Depending on how you use the instrument, you either see the trees, the branches or the leaves.” For this reason, the researchers “coloured” parts of the samples in each case, so to speak, by replacing hydrogen with heavy hydrogen – deuterium. This does not change the structure of the molecules, but it does change their visibility for the neutron probes.The Jülich scientists have a great deal of experience with deuteration and have recently begun offering a proposal-based deuteration service for external users. The researchers also carried out measurements with different scattering contrasts. The appropriate combination of deuteration and scattering contrast made one component of the membrane visible at a time. In order to compile their results, the researchers then had to determine the positional relationships between the components by calculating the scattering contribution of each individual component.
Structure like a sponge
The researchers were therefore able to quantitatively reconstruct the entire structure of the water-covered Nafion membrane using this method, which was applied to Nafion for the first time. Resembling a sponge soaked in water, the structure is self-similar in the orders of magnitude studied, i.e. both the membrane as a whole and its components have a sponge-like structure. Additionally, there are crystalline and disordered structural components. The measurements also showed that the water is homogeneously distributed over a large area of the material. This fits in well with the thesis that the high membrane conductivity of the membrane is based on a well-connected water network.
Original text: Angela Wenzik / FZ Jülich
Original publication:
Yue Zhao et al.; Three-Component Domains in the Fully Hydrated Nafion Membrane Characterized by Partial Scattering Function Analysis; Macromolecules 2021, 54, 9, 4128–4135; Publication Date: April 29, 2021, DOI: 10.1021/acs.macromol.1c00587
- Performance data of the neutron small-angle scattering instrument 2
- Deuteration service of Forschungszentrum Jülich
Contact:
Dr. Aurel Radulescu
Forschungszentrum Jülich
Jülich Centre for Neutron Science (JCNS-FRM-II)
Phone: 089 158860-712
E-Mail: a.radulescu@fz-juelich.de
Press contact:
Angela Wenzik
Wissenschaftsjournalistin
Forschungszentrum Jülich
Phone: 02461 61-6048
E-Mail: a.wenzik@fz-juelich.de
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:



