MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
SANS kinetic study of pH degradable polymer micelles intended for controlled drug delivery
S. K. Filippov1, J. M. Franklin2, P. V. Konarev2, P. Chytil1, T. Etrych1, A. Bogomolova1, M. Dyakonova3, C. M. Papadakis3, A. Radulescu4, K. Ulbrich1, P. Stepanek1, and D. I. Svergun2
1Institute of Macromolecular Chemistry, AS CR, Prague, Czech Republic
2European Molecular Biology Laboratory, EMBL c/o DESY, Hamburg, Germany
3Physik-Department, Technische Universität München, Garching, Germany
4Jülich Centre for Neutron Science (JCNS) at MLZ, Forschungszentrum Jülich GmbH, Garching, Germany
We report on kinetic studies of therapeutically highly potent polymer-drug conjugates consisting of amphiphilic N-(2-hydroxypropyl) methacrylamide (HPMA) based copolymers bearing the anticancer drug doxorubicin (Dox). We performed time-dependent SANS measurements (KWS-2 beamline) after sharp changing pH levels to characterize the drug release and changes in particle size and shape. For most conjugates, nanoparticle growth or decay was observed within a time range of several hours. We concluded that the spacer structure determined the fate of a cholesterol derivative after the pH jump [1].
Hydrolytically degradable polymer micelles intended for controlled drug delivery
Polymer therapeutics is one of the most challenging and promising trends in biomedical technology. Polymer drug carriers based on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers have been developed for the treatment of tumors, with special focus on site-specific delivery and controlled release of anti-cancer agents into tumor tissues or cells. Such interest requires a knowledge of the detailed interior structure of the polymeric nanoparticles and structure evolution during drug delivery. Amphiphilic HPMA-based polymer drug carriers with anticancer drug doxorubicin (Dox) show promising results, exhibiting enhanced tumor accumulation and excellent antitumor activity in the treatment of solid tumors [2].
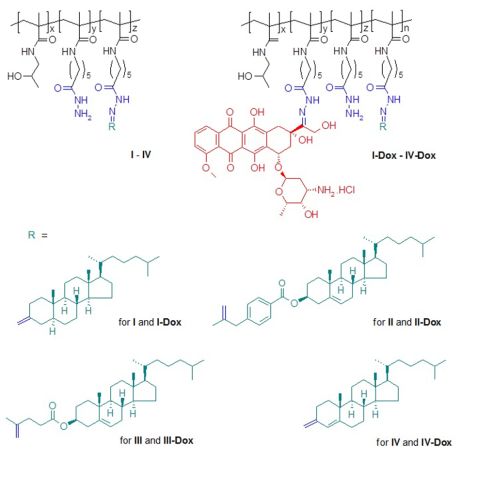
In the present work, the overall properties and internal structure of the new copolymers were investigated as a function of time using SANS/SAXS after a sudden change in pH from 7.2 to 5. Subsequently, the mechanisms of the nanoparticle transformations were analyzed. In contrast to our previous studies [3], fewer hydrophobic pH-responsive HPMA copolymers (fig. 1) were synthesized and used for the experiments. All copolymers had almost the same content of cholesterol derivatives.
SANS kinetic study of hydrolytically degradable polymer micelles
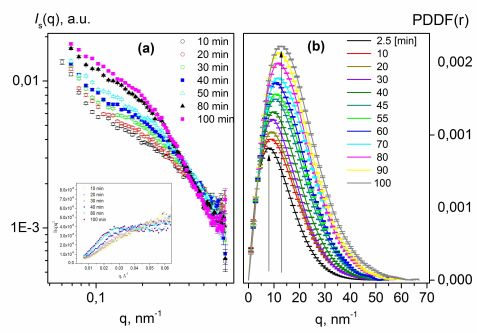
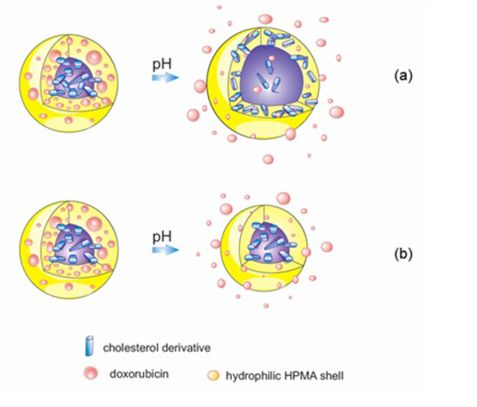
The release of Doxorubicin (Dox), an anti-cancer drug, from amphiphilic N-(2-hydroxypropyl) methacrylamide (HPMA) based copolymers was characterized using time-dependent SANS (KWS-2 beamline) and SAXS measurements after a pH jump simulating the particle transport from blood (pH=7.4) to a tumor environment (pH=5.0). To the best of our knowledge, we were able, for the first time, to monitor the change in properties of a drug delivery carrier with time in conditions mimicking the tumor microenvironment using the SANS/SAXS methods. For most conjugates, nanoparticle growth or decay was observed within a time range of several hours (fig. 2). It was established that the growth/decay rate and the steady-state size of nanoparticles depend on the spacer structure. We concluded that the spacer structure determines the fate of a cholesterol derivative after a pH jump. The elution of Dox from a nanoparticle was traced over time. Fitting results for 5α-cholestan-3-one and Lev-chol spacers implied that cholesterol moieties were continuously escaping from the nanoparticle core and concentrated in the hydrophilic shell (fig. 3). In contrast, the cholest-4-en-3-one spacer seemed to be stable in conditions mimicking tumor cells due to its conjugated double bonds, thus preventing cholesterol escaping. Dox moiety release was only observed for the cholest-4-en-3-one spacer with change in pH (fig. 3). Such findings justify the model proposed in our previous paper. Conversely, micelles composed of Opb-spacers grew more compact with time due to their higher hydrophobicity.
M.D. thanks the MaMaSelf (Master in Materials Science Exploiting Large Scale Facilities) master program for financial support. The MLZ is also acknowledged for beam time allocation. The authors acknowledge financial support from the European Commission under the Seventh Framework Program by means of the grant agreement for the Integrated Infrastructure Initiative N. 262348 European Soft Matter Infrastructure (ESMI). The authors also acknowledge financial support Nr. CZ09-DE06/2013-2014 from the ASCR – DAAD Programme PPP 2013-2014 and from the German Bundesministerium für Bildung und Forschung grant BioSCAT, Contract no: 05K12YE1.
References:
[1] S. Filippov et al., Biomacromolecules 14, 4061 (2013).
[2] P. Chytil et al., Macromol. Chem. Phys. 213, 858 (2012).
[3] S. Filippov et al., Biomacromolecules 13, 2594 (2012).
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: