MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
07.02.2025
Useful in fuel cells or luminescent materials: New compound studied with neutrons

Dr Alexander Mutschke operates the hydrogen detection device "ELEMENTRAC ONH-p2" in the Advanced Materials Laboratory at the Heinz Maier-Leibnitz Zentrum (MLZ). © Bernhard Ludewig, FRM II / TUM
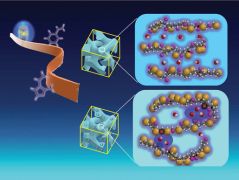
An international team led by Dr Alexander Mutschke has produced a completely new compound with boric acid and hydrogen and elucidated its structure with the help of neutrons. The borate hydride could be used in fuel cells, luminescent materials or catalysis in the future.
It wasn’t exactly a chance hit, admits Alexander Mutschke. When he combined a borate anion BO33- with a hydrogen atom at the Chair of Inorganic Chemistry at the Technical University of Munich, the chemist and his colleague at the time, Dr Thomas Wylezich, had already made dozens of unsuccessful attempts to produce the compound. Mutschke, who now works as a post-doctoral researcher at the MLZ, still enthuses: “This is a rare discovery! There is only one other compound of this kind.” The scientific journal Chemistry – A European Journal has also put the researchers’ publication on the inside cover of its 63rd issue.
Application in catalysers or hydrogen storage
Why did Alexander Mutschke and his colleagues from Australia, Finland, the Czech Republic, Sweden, and Germany go to the trouble of producing and analysing this compound? ‘The idea is to combine the practical properties of both negatively charged ions, borate and hydrogen, in one compound,’ he says. On the one hand, you have the light and often mobile hydrogen, which is important for many catalytic reactions. On the other hand, the unreactive borate, the salt of boric acid, gives the compound a solid and stable framework. ‘This is interesting for hydrogen storage, hydride ion conductors, catalysts or even luminescent materials,’ explains Alexander Mutschke about possible applications.

The article about the new chemical compound has appeared on the inside cover of the scientific publication Chemistry A European Journal.
Structure can only be resolved with neutrons
However, in order to be able to do anything with the compound, its structure must first be understood. And this is where the most spectacular part of the work comes in, as Alexander Mutschke says: ‘The most difficult thing is actually to elucidate the structure.’ This can and could only be done with the help of neutrons. The team carried this out on the Australian counterpart to the MLZ instrument SPODI, the Echidna high-resolution powder diffractometer. Measurements at the German Electron Synchrotron DESY complemented the investigation. ‘As soon as the FRM II delivers neutrons again, further measurements are planned at SPODI to investigate the structure of daughter compounds,’ says Alexander Mutschke.
Original publication:
Alexander Mutschke, Thomas Wylezich, Přemysl Beran, Tobias Hölderle, Volodymyr Baran, Maxim Avdeev, Antti J. Karttunen, Nathalie Kunkel
The Non-Centrosymmetric Borate Hydride Sr4Ba3(BO3)3.83H2.5
Chem. Eur. J. 2024, 30, e202403048 (2024)
doi.org/10.1002/chem.202403048
Associated partners:
In addition to scientists from the Heinz Maier-Leibnitz Zentrum and the Chair of Inorganic Chemistry with Focus on Novel Materials at the Technical University of Munich, researchers from the European Spallation Neutron Source in Lund, Sweden, the Nuclear Physics Institute CAS in Rez, Czech Republic, the German Electron Synchrotron DESY, Australian Nuclear Science and Technology Organisation (ANSTO) in Lucas Heights, Australia and the Department of Chemistry and Materials Science at Aalto University, Finland, were also involved.
Related News
-
28.06.2021
When hydrogen glows red
-
12.06.2020
Hydrogen – but efficiently
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: