MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
07.04.2021
Dancing on dry land: How proteins stay active without water

Dr. Wiebke Lohstroh at the TUM neutron time-of-flight spectrometer TOFTOF, which was able to resolve the motion of the protein-polymer compounds on a time scale of picoseconds. © Andreas Heddergott
Proteins actually need water to perform their function. Looking more closely with neutrons, researchers have observed that it can also be done without water and how the oxygen-transporting muscle protein myoglobin moves in that case. Their results have now been published in the renowned journal Physical Review Letters.
Until recently, it was an unwritten law that proteins need water. Without the liquid element as a lubricant, they cannot move, and thus cannot become active, according to the doctrine. But in 2010 researchers found that multichain molecules, known as polymers, also breathe life into proteins. This is interesting for many applications in medicine and cosmetics. However, it was previously unclear exactly how this works.

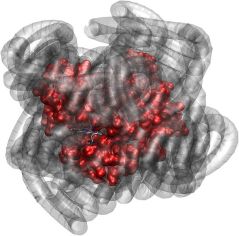
The muscle protein myoglobin is colored red in this graph, the polymers purple. The rising bubbles symbolize oxygen released from myoglobin when the oxygen concentration falls below a threshold. © Warwick Bromley
Film in picosecond resolution
Now, an international team of researchers led by Dr. Martin Weik of the Institut de Biologie Structurale at the University of Grenoble, France, have used neutrons at the Heinz Maier-Leibnitz Zentrum to see how proteins with polymers move compared to aqueous solution. “We had measured this roughly for the first time ten years ago (press release Aug. 2, 2012). At that time, we were able to show that the protein moves and thus still functions, even though the water is gone.” explains Martin Weik. “Now we are trying to understand exactly how these movements take place.” For the more detailed measurements on the oxygen-transporting muscle protein myoglobin, the group used the SPHERES backscattering spectrometer as before, and this time also carried out measurements at the TOFTOF time-of-flight spectrometer. “Here we can follow the movements of the myoglobin and polymers, which are distinctly labeled, down to a time scale of picoseconds,” says Dr. Wiebke Lohstroh, instrument scientist and leading researcher at the Technical University of Munich’s TOFTOF instrument. That is because neutrons reveal the motions of hydrogen atoms more clearly than those of heavier deuterium, which is used as a marker. The extended measurements at SPHERES have also made a crucial contribution: “With the high-resolution spectra now measured, it is possible to observe various motion processes directly at the molecular level,” says Dr. Michaela Zamponi, an instrument scientist at SPHERES from Forschungszentrum Jülich.
Polymer mimics movement of water
“With the results, we make a concrete suggestion on what to improve in the polymer to make the protein even more active,” says Martin Weik. “Surprisingly, we found that the polymer mimics movements of water.” This keeps the dry protein biologically active, but certain movements are suppressed. This helps the researchers explain why the protein is less active when the water is replaced by polymers. With a little fine-tuning, however, it is possible to make the protein more active.
Heart patients benefit
The knowledge gained from this study is useful for the development of next-generation cell therapies for treating cancer or regeneration after heart attacks. The aim of the scientists is to use protein-polymer hybrids in cell therapies, e.g., to help heart attack patients recover by regenerating healthy heart tissue. The advantage of the waterless protein-polymer compounds is that they prolong the survival of the patient and promote the settlement of the transplanted cells.
Original publication:
Diffusive-like motions in a solvent free protein-polymer hybrid.
Giorgio Schirò, Yann Fichou, Alex P. S. Brogan, Richard Sessions, Wiebke Lohstroh, Michaela Zamponi, Gerald J. Schneider, François-Xavier Gallat, Alessandro Paciaroni, Douglas J. Tobias, Adam Perriman, Martin Weik. In: Phys. Rev. Lett. Vol. 126, No. 8 (2021)
DOI: 10.1103/PhysRevLett.126.088102
More information:
The research also involved scientists from the following institutions: University of Bristol, UK, School of Biochemistry, Bristol, UK, King’s College London, UK, Louisiana State University, USA, University of California, USA, Università degli Studi di Perugia, Italy.
Related News
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: