MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
22.03.2021
Conjuring colors out of cylinders
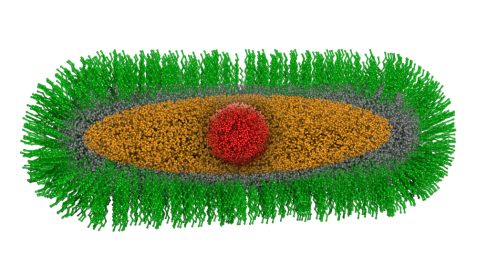
Schematic structure of the "green nanorods": the spherical core of cadmium selenide (red) is surrounded by a crystalline ellipsoidal cadmium sulfide (orange) and a crystalline cylindrical zinc sulfide layer (gray). This complex "core-shell-shell" particle is stabilized by an organic ligand shell (green). © Reiner Müller / FRM II, TUM
Nanoparticles can be found in pharmaceuticals, catalysts, and even TVs. Researchers at the chemical company Merck, the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), and the University of Duisburg-Essen (UDE) used neutrons at MLZ to study colloidal nanoparticles. They are particularly well suited for converting electrical signals into light or, vice versa, light into electricity.
Green nanorods promise a rich pallet of applications: For example, they are already used in screens of modern QLED TVs. They consist of cadmium selenide with cylindrical protective shells, where the outermost stabilization layer is organic and whose hydrocarbon chains reach outward like tiny hairs. The nanoparticles owe their name to their strongly pronounced green glow. “The advantage of such nanoparticles is that their optical properties, and thus the color of the glow, can be tuned by their size. Moreover, unlike their organic counterparts, inorganic particles cannot fade.” says Prof. Dr. Tobias Unruh from the Institute for Crystallography and Structural Physics of the FAU.

In the left beaker the Nanorods are solved. Their green color is visible, from which they derive their name. © FAU
Protective shells preserve the nanoparticles’ properties
Researchers can tune special properties of nanoparticles by changing not only their chemical composition and structure but also their size, shape, and interfacial properties. The problem is that nanoparticles lose some or all their special properties when they encounter molecules in their environment. The protective shells should prevent this and at the same time control the contact between them. However, currently there is a lack of methods and models to predict the production of such complex nanoparticles with tailored properties. To this end, the research team has closely examined the structure of already known nanoparticles.
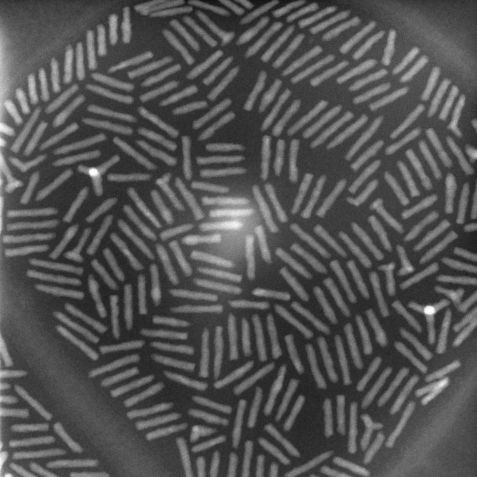
Nanocylinders under the electron microscope. This allows to determine the dimensions of individual nanocylinders. With neutrons the researchers shine trough a much larger sample section and obtain averaged values of several cylinders. © FAU
Neutrons show the stability of the shell
Organic molecules are added during the production of the nanoparticles, some of which are required for the nanoparticle synthesis and some of which are incorporated into the shell itself. In the end, the remnants of the organic molecules must be washed out without removing the shell itself. Until now, it was unclear how much the shell would suffer under the washing. “With the help of neutrons, we were able to take a closer look at the organic shell,” says Dr. Sebastian Busch, instrument scientist at MLZ’s SANS-1 instrument, where these nanoparticles were studied. Neutrons are ideal for this study because, when hydrogen is replaced with deuterium in the solvent, it is quite easy to distinguish the shell from its surroundings. The researchers used the neutron measurements to show that the washing process not only leaves the shell undamaged, but actually strengthens it.
Original publication:
Lin, W., Greve, C., Härtner, S., Götz, K., Walter, J., Wu, M., Rechberger, S., Spiecker, E., Busch, S., Schmutzler, T., Avadhut, Y., Hartmann, M., Unruh, T., Peukert, W., Segets, D., Unraveling Complexity: A Strategy for the Characterization of Anisotropic Core Multishell Nanoparticles. Part. Part. Syst. Charact. 2020, 37, 2000145.
https://doi.org/10.1002/ppsc.202000145
More Information:
Institute of Particle Technology of the FAU: https://www.lfg.tf.fau.de/institute/
Institute for Crystallography and Structural Physics der FAU: https://www.icsp.nat.fau.eu/
Researchgroup of Prof. Doris Segets (UDE): https://www.uni-due.de/ivg/segets-group/
Merck KGaA, Electronics: https://www.merckgroup.com/en/company/who-we-are/electronics.html
Related News
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:



