MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
11.02.2021
Captured lithium

The two authors Dr. Martin Mühlbauer (l.) and Dr. Anatoliy Senyshyn at the instrument STRESS-SPEC with a commercially available lithium-ion battery. © A. Heddergott / TUM
In our smartphones, our computers and in our electric cars: We use rechargeable lithium-ion batteries everywhere. But their capacity drops after a while. Now a German-American research team has investigated the structure and functionality of these batteries using neutron diffraction: They discovered that the electrolyte fluid’s decomposition products capture mobile lithium in the battery and that the distribution of lithium within the cell is surprisingly uneven.
The outstanding characteristics of the lithium-ion battery have changed our everyday lives as only very few other inventions have. However, over time various effects occur which gradually reduce the great storage capability of these batteries.
At the Technical University of Munich’s Research Neutron Source Heinz Maier-Leibnitz (FRM II) Dr. Anatoliy Senyshyn, instrument scientist at the high-resolution powder diffractometer SPODI, used neutron scattering to investigate the cause of these effects in cylindrical lithium-ion batteries.
Together with other scientists he is searching for answers to fundamental questions on the structure and behavior of rechargeable lithium-ion batteries: Why does the available capacity drop over time? How is the lithium distributed in the battery?
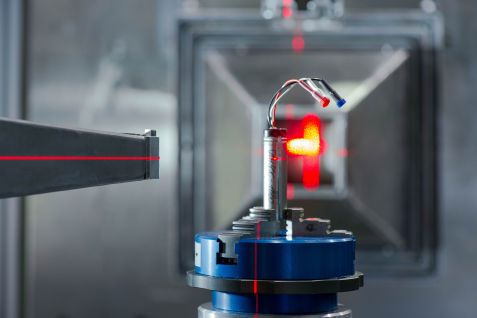
Lithium-ion cell in the slide of the STRESS-SPEC diffractometer at FRM II, which investigates questions in materials science. © A. Heddergott / TUM
Why use neutrons to investigate a rechargeable battery?
Observing a lithium-ion cell’s interior during charging and discharging, for example the decomposition of the electrolyte or the distribution of the lithium, is very difficult from outside of the cell due to the high reactivity of the cell components with oxygen and air humidity.
Neutrons are particularly sensitive with regard to lighter elements such as hydrogen and lithium. Therefore they can render the lithium inside a cell visible, making investigations possible under actual operational conditions.
Another advantage of working with neutrons is that they can be used in non-destructive testing. Thus for example the researchers can observe internal battery processes from outside without intervening in the delicate system.
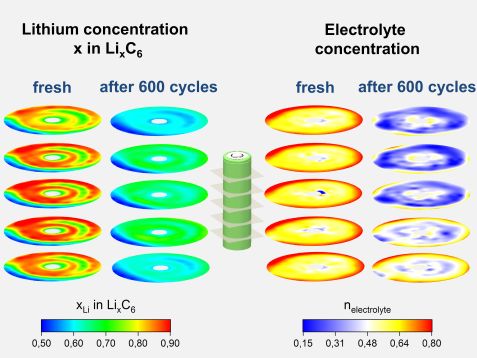
Color-coded concentration of lithium and electrolyte in a fresh and aged (600 charge/discharge cycles) lithium-ion cell. © A. Senyshyn / TUM
Why does battery capacity sink?
Lithium-ion cells produce electricity as lithium atoms release electrons which then flow through a connected device. At the same time, inside the cell a lithium ion moves from one electrode to the other; this means there are always only as many electrons available as there are lithium ions. When the rechargeable battery loses capacity, it can also be said that the lithium “gets lost”. But where does it go?
The neutron scattering experiments at the instruments STRESS-SPEC and SPODI indicated a linear relationship between the loss of mobile lithium ions and the decomposition of the electrolyte, which occurs for example as unwanted side reaction during charging.
The products resulting from the electrolyte decomposition trap lithium atoms which are then no longer available as mobile lithium to be exchanged between the two electrodes. As a result, the battery loses capacity: It ages.

Neutrons make it possible to look non-destructively inside the lithium-ion batteries and see the distribution and amount of lithium in the graphite anode. In the background is the structure of the graphite that has incorporated lithium. © Reiner Müller
How is the lithium distributed?
Until now theoretical models, calculations and measurements usually assume an even distribution of lithium in these batteries. However, the investigations indicate that the lithium is very unevenly distributed from the very beginning; and the inhomogeneity rises over time.
Modelling of lithium-ion cells can be significantly improved when developers take this uneven lithium distribution into account. In addition, statements can be made about the storage ability of the lithium-ion cell based on the lithium distribution. These results are an important basis for making more efficient rechargeable batteries with longer service lives and higher performance in the future.
Publications:
Dominik Petz, Martin J. Mühlbauer, Alexander Schökel, Klaus Achterhold, Franz Pfeiffer, Thilo Pirling, Michael Hofmann,
and Anatoliy Senyshyn
Heterogeneity of graphite lithiation in state-of-the-art cylinder-type Li-ion cells
Batteries and Supercaps, Oct. 28, 2020 – DOI: 10.1002/batt.202000178
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/batt.202000178
M.J. Mühlbauer, D. Petz, V. Baran, O. Dolotko, M. Hofmann, R. Kostecki, A. Senyshyn
Inhomogeneous distribution of lithium and electrolyte in aged Li-ion cylindrical cells
Journal of Power Sources, 475 (2020) 228690 – DOI: 10.1016/j.jpowsour.2020.228690
https://doi.org/10.1016/j.jpowsour.2020.228690
More information:
The work was funded by the Heinz Maier-Leibnitz Zentrum (MLZ), the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF) and the Bavaria California Technology Center (BaCaTeC), as well as by the U.S. Department of Energy, as part of its research activities on advanced battery materials.
In addition to scientists at the Heinz Maier-Leibnitz Zentrum and the Technical University of Munich Department of Physics, researchers from the Karlsruhe Institute of Technology, Iowa State University’s Ames Laboratory (USA) and Lawrence Berkeley National Laboratory (USA) were also involved in the publication. The measurements were carried out at the Heinz Maier-Leibnitz Research Neutron Source (FRM II) of the Technical University of Munich, the Institute Laue-Langevin (ILL) in Grenoble (France) and at the German Electron Synchrotron (DESY) in Hamburg.
Contact:
Dr. Anatoliy Senyshyn
Forschungs-Neutronenquelle Heinz Maier-Leibnitz (FRM II) and
Heinz Maier-Leibnitz Zentrum (MLZ)
Technische Universität München
Lichtenbergstr. 1, 85748 Garching
Tel.: +49 89 289 14316
E-Mail: anatoliy.senyshyn@frm2.tum.de
Related News
-
04.02.2020
More battery,more energy revolution
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: