MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
08.01.2024
Position swapping in cathodes: Why batteries lose performance
When developing new generations of batteries, researchers are challenged by various physical effects that reduce the storage capacity. Using state-of-the-art neutron scattering, scientists from FRM II and Australia have investigated one of these effects: the jumping of lithium ions in the cathodes of commercial batteries.
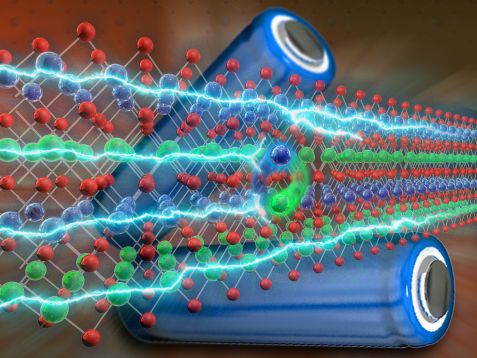
Lithium (green) and nickel atoms (blue) swap places in lithium-nickel-oxide batteries, thus reducing battery performance. © Reiner Müller, FRM II
In the search for more efficient, longer-lasting, and safer battery technologies, researchers must first understand what makes a good battery. State-of-the-art batteries face various challenges. These can be partly explained by the cathode material used. In the past, cathodes made of lithium cobalt oxide (LCO), which already had high energy densities, were mainly used. However, the cobalt oxide contained has a high reactivity, which, in the worst case, leads to overheating and ignition of the cell and reduces the maximum number of charging cycles. Other disadvantages include high production costs and the limited availability of cobalt worldwide.
From jumping atoms
“Nickel was considered a promising alternative for cobalt, but structural defects also occur in lithium-nickel-oxide cathodes, which quickly reduce capacity and energy density,” explains Tobias Hölderle, a doctoral student at the Technical University of Munich and the MLZ. He is the first author of the publication.
One dominant defect is cation mixing, in which the positively charged nickel and lithium ions can swap their positions in the crystal lattice due to their similar atomic radius.
“These structural defects are a burden for the battery. During charging and discharging, nickel ions can sit firmly in the lithium positions, blocking the diffusion paths of the surrounding lithium. This reduces battery performance and the structural stability of the atomic lattice,” says Hölderle.
Batteries installed in electric cars
To improve structural stability, manufacturers do not entirely replace the cobalt atoms in the cathodes of their battery products with nickel. Instead, most use a material comprising a proportion of cobalt, nickel, and transition metals such as aluminum or manganese. Empirically, the manufacturers have found mixed ratios between these elements in which the battery’s service life, costs, and safety are within an acceptable range. Batteries of this type are also installed in the first generation of the Tesla Model S.
While the existence of cation mixing for cells with nickel-manganese-cobalt (NMC) has already been proven, the occurrence in nickel-cobalt-aluminum cathodes (NCA) has remained controversial to this day.

Tobias Hölderle is a doctoral student at TUM and the FRM II and is researching effects that occur in batteries. © Bernhard Ludewig
Neutrons peer into batteries
Tobias Hölderle and his co-authors examined commercial NCA cathodes in different states of charge using neutron diffraction at the SPODI instrument at the MLZ and the ECHIDNA instrument of the Australian Centre for Neutron Scattering (ACNS) in Sydney to gain insight into the atomic crystal structure of the cathodes.
Neutrons are particularly suitable for research on batteries, as they are scattered disproportionately by comparatively light lithium atomic nuclei and thus make the position of the lithium in the data particularly visible. Neutrons are, therefore, essential for observing the exchange of lithium and nickel ions in the cathode structure.
No cation mixing in NCA cathodes
The researchers used their measurements to analyze the NCA cathode structure and cation mixing. This shortens the atomic distances during the charging process. The lithium-ion concentration fell in proportion to the state of charge, but they did not observe any lithium-nickel exchange despite the high nickel content.
Tobias Hölderle summarizes the results: “We conclude from this that, in contrast to the NMC cathode material, there is no cation mixing in the NCA cathodes.” In parallel, the group carried out electrochemical measurements on the cell, the results of which support those of the neutron measurements.
Original publication:
T. Hölderle, M. Monchak, V. Baran, O. Dolotko, S. Bette, D. Mikhailova, A. Voss, M. Avdeev, H. Ehrenberg, P. Müller-Buschbaum, and A. Senyshyn
The structural behavior of electrochemically delithiated LixNi0.8Co0.15Al0.05O2 (x<1) battery cathodes
Journal of Power Sources 564 (2023) 232799
10.1016/j.jpowsour.2023.232799
More information:
This work received financial support from the Heinz Maier-Leibnitz Zentrum (Technical University of Munich), the German Federal Ministry of Education and Research (BMBF projects 05K16VK2 and 05K19VK3), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, projects EH 183/15-1, SE 2376/1-1]). This work contributes to research at CELEST (Center for Electrochemical Energy Storage Ulm-Karlsruhe).
In addition, participating authors came from the Institute for Applied Materials (IAM) at the Karlsruhe Institute of Technology (KIT), the Deutsches Elektronen Synchrotron (DESY) in Hamburg, the Max Planck Institute for Solid State Research (MPI-FKF) in Stuttgart and the Leibniz Institute for Solid State and Materials Research Dresden (IFW).
Contact:
Dr. Anatoliy Senyshyn
Research Neutron Source Heinz Maier-Leibnitz (FRM II) and Heinz Maier-Leibnitz Zentrum (MLZ)
Instrument SPODI
Phone: +49 89 289 14316
E-Mail: anatoliy.senyshyn@frm2.tum.de
Related News
-
06.02.2023
Neutron beam for better batteries
-
19.07.2022
Engineered disorder
-
08.06.2022
On the road to the super-battery
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media: