MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
18.04.2024
Efficient iron scavengers
The cyanobacterium Prochlorococcus is the smallest and most abundant photosynthetic organism in the world. It has found a particularly efficient way to absorb and store vital iron. Using neutron, X-ray, and synchrotron experiments, an international team of researchers has visualized the molecular mechanism for the first time.
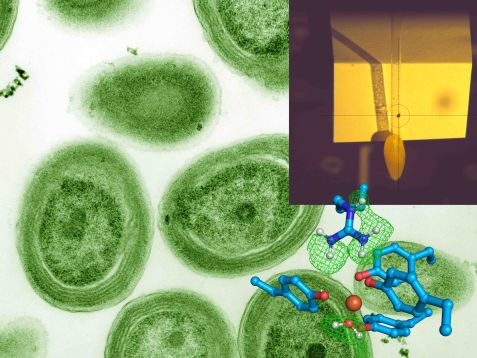
The research team analyzed the bacterium Prochlorococcus (background). In the crosshairs of the Biodiff instrument (top right) is one of the crystals of the FutA protein that led to the structure shown below. Iron (reddish red) is shown in the center. The blue amino acid side chains and a water molecule (bottom right) bind to the iron. The neutron experiment showed the hydrogen atoms' location, encased in a lattice of green lines. © Dr. Tobias Schrader, Forschungszentrum Jülich; Luke Thompson from Chisholm Lab and Nikki Watson from Whitehead, MIT, CC0, via Wikimedia Commons (Hintergrundbild)
The sea is the largest ecosystem on Earth. Organisms living in the sea, which carry out photosynthesis, produce around half of the oxygen on Earth. At the same time, they are an important carbon sink in the fight against climate change. Every year, they bind a total of 45 gigatonnes of carbon. Photosynthetic bacteria are one of the main players here, and the cyanobacterium Prochlorococcus holds several records in this field: with a diameter of half a micrometre – the equivalent of half a millionth of a metre – it is the smallest photosynthetic organism on Earth. It produces four gigatonnes of sequestered carbon per year, which corresponds to the annual net primary production of global agriculture. Its photosynthetic activity, however, depends on iron, which is only available to a limited extent in the ocean.
One protein binds two different forms of iron
Biologically relevant iron can occur in nature in two different oxidation states, as divalent or trivalent iron ions, Fe 2+ and Fe 3+ – i.e., as a charged atom that has released two or three electrons. Most organisms, including cyanobacteria, usually have two different proteins to absorb these different oxidation states. This is different for Prochlorococcus, which can bind both forms of iron with a single protein.
Compact genome in tiny bacteria
The researchers suspect this dual function is an important reason for ecological success and is linked to the compact genome of Prochlorococcus. As this marine bacterium is so tiny, it only has a greatly reduced genome and, therefore, has to manage with a limited number of genes that could be translated into different proteins.

Dr. Tobias Schrader, instrument scientist at BioDiff, at the Heinz Maier-Leibnitz Zentrum. © Bernhard Ludewig, FRM II / TUM
Molecular switch
An international research group headed by Prof. Dr. Ivo Tews from the University of Southampton has now demonstrated for the first time the mechanism by which Prochlorococcus absorbs the two different forms of iron. The researchers discovered a molecular switch that enables the bacterium to switch back and forth between binding divalent and trivalent iron ions. The “FutA protein” also has another function. In the bacterium, it is not only responsible for the uptake, but also for the protection of the iron that has already been absorbed.
Combination of X-rays, neutrons, and spectroscopy
The discovery was made possible by a novel combination of experiments. The researchers used X-rays, neutron beams, and light absorption to understand the iron binding process. They also used X-rays to switch between the two forms of iron. In several experiments carried out at the Diamond Light Source and also at the RIKEN SPring-8 Center in Japan, the researchers determined the position of the atoms – with the exception of the hydrogen atoms. Their arrangement could only be made visible with the help of neutron beam experiments.
Neutrons show a state of charge in the iron
Experiments with neutron beams at the research neutron source Heinz Maier-Leibnitz (FRM II) in Garching thus helped to understand the iron’s charge state. “The BIODIFF diffractometer, which Forschungszentrum Jülich operates together with the Technical University of Munich, is designed for determining the position of hydrogen atoms in such large molecules,” explains Dr. Tobias Schrader from the Jülich Centre for Neutron Science (JCNS). Based on this, the researchers calculated the charges of the amino acid side chains in the protein around the iron. These, in turn, allow conclusions to be drawn about the charge of the iron.
“Using a nuclear reactor to see the hydrogen atoms was really exciting for me. Even the hydrogen atoms in the water were clearly visible,” says Rachel Bolton, a doctoral student at the University of Southampton, who carried out the experiments in Garching.
Original text:
University of Southampton / Forschungszentrum Jülich
Original publication:
Rachel Bolton, Moritz M. Machelett Jack Stubbs, Danny Axford, Nicolas Caramello, Lucrezia Catapano, Martin Malý, Matthew J. Rodrigues, Charlotte Cordery, Graham J. Tizzard, Fraser MacMillan, Sylvain Engilberge, David von Stetten, Takehiko Tosha, Hiroshi Sugimoto, Jonathan A. R. Worrall, Jeremy S. Webb, Mike Zubkov, Simon Coles, Eric Mathieu, Roberto A. Steiner, Garib Murshudov, Tobias E. Schrader, Allen M. Orville, Antoine Royant, Gwyndaf Evans, Michael A. Hough, Robin L. Owen, and Ivo Tews
A redox switch allows binding of Fe(II) and Fe(III) ions in the cyanobacterial iron-binding protein FutA from Prochlorococcus
PNAS 121 (12) e2308478121 (2024)
DOI: 10.1073/pnas.2308478121
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:



