MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
19.05.2023
A protective layer for batteries
The storage capacity, safety, and service life of rechargeable batteries must significantly increase to produce for example more reliable electric cars with higher mileage. A lithium-metal battery can offer much higher storage than a lithium-ion battery but there is concern about the safety issue. A protective coating of the anodes of lithium metal batteries shows great potential here for future products.
If the graphite anode of a lithium-ion battery is replaced with a metallic lithium anode, the theoretical energy density of the resulting lithium-metal battery (LMB) is about ten times the previous maximum.
Problem solved, then?
“No, unfortunately, effects also occur with LMBs that compromise safety, and efficiency after repeated charging and discharging,” explains Dr. Neelima Paul, a researcher at MLZ. The exposed lithium anode is in constant contact with the reactive electrolyte that fills the space between the two electrodes. Electrochemical reactions with the electrolyte and the resulting uneven accumulation of lithium metal deposits on the surface of the metal anode lead over time to misshapen outgrowths. These reduce the efficiency of the battery cells as they grow because less electrochemically active or cyclable Li is available but can also lead to a short circuit that renders the cell unusable.
To prevent this, Neelima Paul together with scientists from Stanford University (USA), Forschungszentrum Jülich, and MLZ colleagues Prof. Dr. Peter Müller-Buschbaum and Dr. Ralph Gilles, investigated polymer protective layer coatings on anodes in LMBs.
Polymers follow lithium ions like seaweed follows the wave
Polymers are flexible chains of repeating molecules whose mechanical properties can be influenced by variations in their components. They are easy to manufacture and are popular in science and engineering as coatings.
In previous studies, researchers have already shown that a polymer coating leads to more uniform growth of the lithium metal of the anode. What remained unclear, however, was what effect the dynamics of the polymers had on this effect.
In their new study, the researchers looked at polymers with units that bind more strongly to each other, resulting in a rigid coating, compared to weaker-binding units that exhibit greater flexibility. The theory is that more flexible (or softer) polymer chains always follow the flow of lithium ions, like seaweed in a wave. As a result, they always cover the areas on the anode to which the flow is currently highest, thus temporarily shielding them. Further growth at this point is thus inhibited.
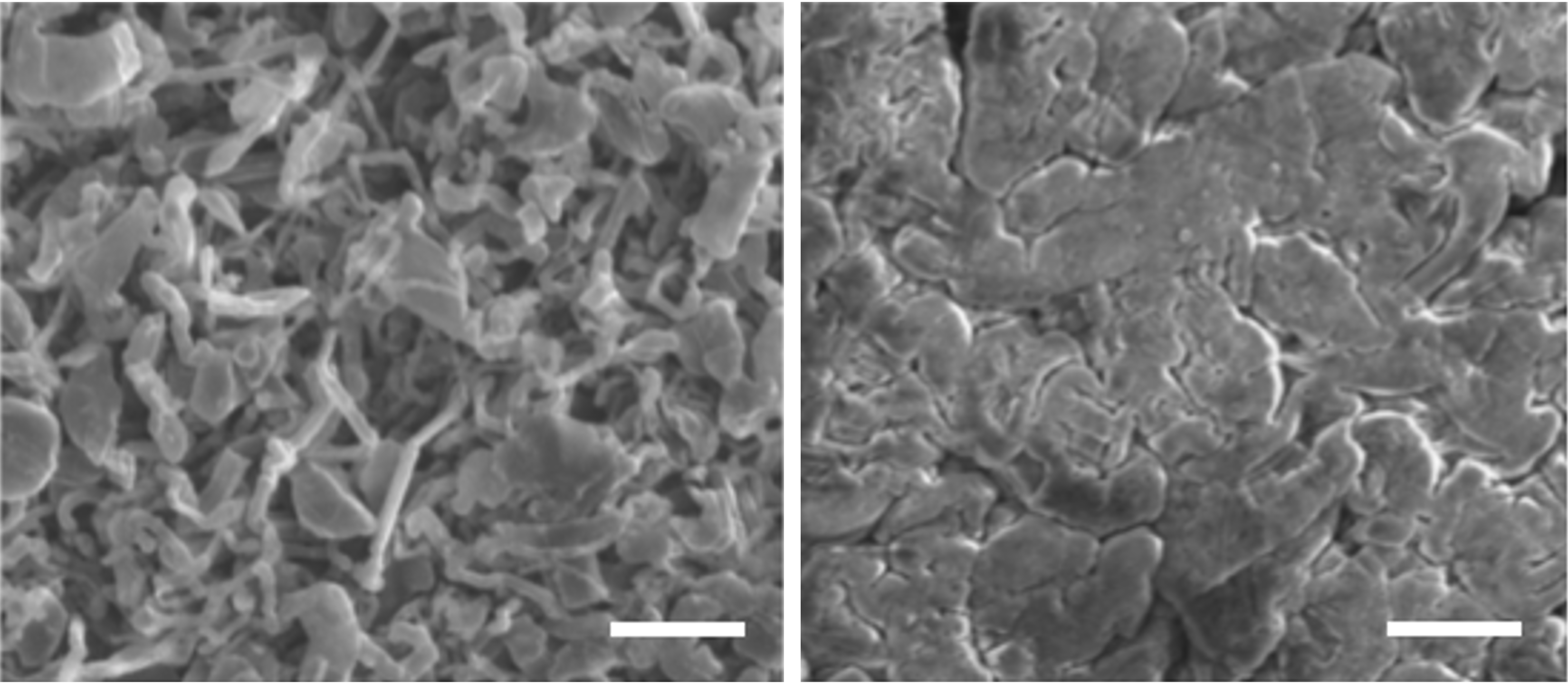
Comparison between the uncoated anode surface (l.) and the smoother surface with a polymer coating (r.) after several charging cycles. © Huang et al.
A look at the interior with X-rays
“But first we need to examine the structure-property relationship of these polymers’’ explains Dr. Neelima Paul. The scientist examined a polymer series having similar chemistry but varying mechanical strengths with X-rays to investigate the evolution of their inner structure and morphology, depending on their composition. “Two very good methods that are available to us there are SAXS and WAXS.”
Both SAXS and WAXS – the abbreviations stand for small- and wide-angle X-ray scattering – are very useful, established methods for both soft matter and material science research. Neelima Paul bombarded polymer samples at the TU Munich with X-rays and then looked at the ring-shaped patterns created by the scattering on the detectors behind them. From the radii, widths, and intensity distribution of these rings, she was able to gain insights into mobility, spacing, and orientation of the units within the polymers relative to one another and concluded that the mechanically weak polymers are indeed more flexible than the mechanically strongest polymer and have a random orientation of their units.
The softer, the more uniform
Thereafter, the researchers exposed LMBs with and without the different polymer coatings on the anode to dozens of charge-discharge cycles and observed their performance and morphology as time progressed. In accordance with expectations, they were able to determine that the more flexible polymers, which bonded together more weakly, actually caused the lithium growth to occur more uniformly at the anode surface. “We found an optimal composition of the polymers with which we were always able to obtain a homogeneous Lithium deposition and the best cycling performance and highest efficiency“, Neelima Paul adds.
These findings provide valuable design principles that will be applied in further research and possible commercial use of optimized polymer coatings on anodes.
Original publication:
Z. Huang, S. Choudhury, N. Paul, J. H. Thienenkamp, P. Lennartz, H. Gong, P. Müller-Buschbaum, G. Brunklaus, R. Gilles, and Z. Bao
Effects of Polymer Coating Mechanics at Solid-Electrolyte Interphase for Stabilizing Lithium Metal Anodes
Adv. Energy Mater. 2022, 12, 2103187
DOI: 10.1002/aenm.202103187
More information:
This research was supported by funds from the U.S. Department of Energy (DOE), the German Federal Ministry of Education and Research (BMBF, grants 13XP0224A & 03XP0224C), the National Science Foundation (ECCS-1542152), and the German Research Foundation (DFG).
Contact:
Zhenan Bao, Ph.D.
Department of Chemical Engineering
Stanford University
Stanford, CA 94305, USA
E-mail: zbao@stanford.edu
Dr. Ralph Gilles
Heinz Maier-Leibnitz Zentrum (MLZ), TU Munich
Head of Advanced Materials
Phone : +49 89 289 14665
E-mail: ralph.gilles@frm2.tum.de
Related News
-
04.02.2020
More battery,more energy revolution
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:






