MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:

MLZ (eng)
Lichtenbergstr.1
85748 Garching
06.11.2023
A new phase in the battery
How do Li-ion batteries become more environmentally friendly, cheaper and more powerful? An international research team investigated this question. With the help of neutron and X-ray radiation, they examined the crystalline structure of a new cathode material and discovered a third, previously unknown structural phase.
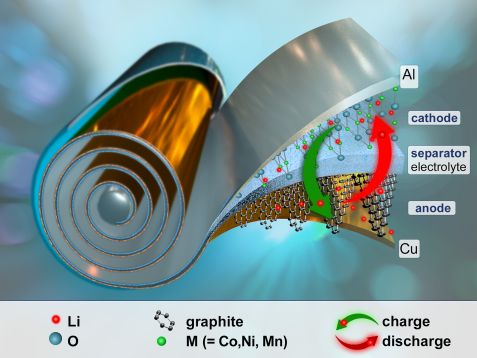
Principle of a lithium-ion battery. © Reiner Müller, FRM II / TUM
A lithium-ion battery consists of a positive cathode and a negative anode, the two so-called electrodes. When charging the battery, the lithium ions flow from the cathode to the anode, where they accumulate. This creates an electrochemical potential within the cell. When discharging, the process is reversed and current flows. The lithium ion content of an electrode is therefore constantly changing.
Substitute for toxic cobalt
Most cathodes contain the toxic cobalt, which is often mined under environmentally unfriendly conditions and fluctuates greatly in price. Manganese-based cobalt-free spinels are a cheap alternative for cathodes in lithium batteries. Spinels consist of metallic cations and oxygen anions. In addition, spinels have a high operating voltage, allowing them to deliver more power. They also enable a lower end-of-discharge voltage, which indicates the voltage down to which the battery can be discharged without being damaged. As a result, the cell releases more energy per charging cycle.

Dr. Neelima Paul at the X-ray diffractometer of the physics laboratory at the Heinz Maier-Leibnitz Zentrum. © Bernhard Ludewig, FRM II / TUM
More power, but higher wear
If the lithium content of the spinel is increased, its energy density increases. This allows the battery to store more energy and perform better with the same weight. This means, for example, a greater range for electric cars. However, the lithium-rich spinel cell wears out surprisingly quickly at low voltage. It is not yet known why the battery wears out faster. Therefore, Dr. Neelima Paul from the MLZ and her colleagues from the Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW) and the Czech Academy of Sciences (CAS) analyzed the structure of the spinel in more detail, focusing in particular on the lower voltage range.
X-rays and neutrons in combination
When the chemical composition of the spinel changes, its crystal structure also changes. Therefore, the material goes through different phases when charging and discharging the battery. At the MLZ, Dr. Neelima Paul used X-ray diffraction to identify these phase changes during battery operation. The team also studied the phenomenon using neutron diffraction at the Nuclear Physics Institute CAS in Rez, Czech Republic. “Since X-rays are scattered by the electrons and neutrons by the nuclei, the two methods provide complementary information about the structure of a crystal,” explains Neelima Paul. “If we examine the material in battery operation, we can also observe short-lived intermediate phases. With the neutrons, we can also determine the exact position of the lithium atoms.”
Three phases instead of two
“During the lithiation of the cathode in the low-voltage range, we observed and investigated a third previously unknown phase,” says Neelima Paul. It was previously known that the spinels change in equilibrium from one phase to another in the normal operating voltage range. Using their measurement technique, the team studied a short-lived third phase that occurs when a lot of lithium has already accumulated on the cathode. It forms at low voltage when the battery is charged and discharged too quickly, as its crystalline structure is then energetically favored. The phase affects the reaction mechanisms that take place at the electrodes, causing the battery voltage to drop faster. This has a negative effect on the life of the battery. With the results of the study, the researchers can better understand why the cells become short-lived at low voltage. Now that the researchers have identified the problem, scientists can work on solutions in the future to avoid this third phase of the batteries and thus extend their lifespan.
Original publication:
Nicola M Jobst, Neelima Paul, Premysl Beran, Marilena Mancini, Ralph Gilles, Margret Wohlfahrt- Mehrens and Peter Axmann
Dynamic Structure Evolution of Extensively Delithiated High Voltage Spinel Li1+xNi0.5Mn1.5O4 x < 1.5
J.Am. Chem. Soc. 145, 8, 4450 (2023)
https://doi.org/10.1021/jacs.2c09621
Further information:
The project was financially supported by the Federal Ministry of Education and Research (BMBF).
The neutron diffraction measurements were performed in the CANAM infrastructure of (Nuclear Physics Institute) NPI CAS, Rez (Czech Republic).
Related News
-
22.05.2023
A protective layer for batteries
-
04.02.2020
More battery,more energy revolution
MLZ is a cooperation between:
 > Technische Universität München
> Technische Universität München > Helmholtz-Zentrum Hereon
> Helmholtz-Zentrum Hereon
 > Forschungszentrum Jülich
> Forschungszentrum Jülich
MLZ is a member of:
 > LENS
> LENS > ERF-AISBL
> ERF-AISBL
MLZ on social media:






